Abstract
Aims: ST-elevation acute coronary syndrome (STE-ACS) is characterised by compromised blood flow at the epicardial and microvascular levels. Endothelin-1 (ET-1) is a mediator of microvascular dysfunction and adverse cardiac remodelling. We hypothesised that administration of an endothelin type A (ETA) receptor antagonist (BQ-123; Clinalfa, Läufelfingen, Switzerland) may protect microvascular function.
Methods and results: In this proof-of-concept, randomised, double-blind, placebo-controlled trial, patients with posterior-wall STE-ACS (n=57) were randomly assigned to receive intravenous BQ-123 at 400 nmol/minute or placebo over 60 minutes, starting at the onset of primary percutaneous coronary intervention (PCI). Time to myocardial contrast wash-in of the infarcted segment assessed by first-pass perfusion cardiac magnetic resonance imaging was the primary efficacy endpoint. Secondary endpoints included enzymatic infarct size and left ventricular ejection fraction (LVEF). In patients randomised to BQ-123 we observed shorter microvessel perfusion delays six days after PCI (1.8 sec [0.7-3.4] versus 3.3 sec [2.3-5.4] in placebo-treated patients, p=0.005). The treatment group demonstrated smaller enzymatic infarct sizes (p=0.014). All patients were alive at six months, with an LVEF of 63% (58-69) in patients randomised to BQ-123 and 59% (51-66) in placebo-treated patients (p=0.047).
Conclusions: Administration of an ETA receptor blocker during primary PCI in patients with STE-ACS is safe and may improve tissue-level perfusion and LVEF.
Abbreviations
AMI: acute myocardial infarction
CK: creatine phosphokinase
ERA: endothelin receptor blocker
ET: endothelin
IRA: infarct-related coronary artery
LVEDV: left ventricular end diastolic volume
LVEF: left ventricular ejection fraction
LVESV: left ventricular end systolic volume
MACE: major adverse cardiac events
MRI: magnetic resonance imaging
NT-proBNP: amino-terminal pro-B-type natriuretic peptide
PCI: percutaneous coronary intervention
STE-ACS: ST-elevation acute coronary syndrome
TIMI: Thrombolysis In Myocardial Infarction
Background
Timely reperfusion is critical to limit cellular and vascular injury in acute myocardial infarction (AMI)1; however, the benefits may be attenuated by reperfusion injury of endothelial cells and cardiomyocytes2, and compromised microvascular reflow3.
Endothelin-1 (ET-1) is a potent vasoconstrictor peptide, abundant in unstable human coronary lesions4, acute coronary thrombus5 and markedly up-regulated in reperfused ischaemic myocardium6,7. ET-1 plasma levels predict no-reflow immediately after percutaneous coronary intervention (PCI)8, as well as long-term clinical outcome after AMI9.
Controversy exists regarding the role of ET receptor blockers (ERA) in AMI models. In a number of animal models10,11 ERA have been shown to reduce infarct size, while there are also reports of unfavourable effects of early use of ET receptor antagonists in AMI12,13. Based on previous data illustrating that ET triggers microcirculatory dysfunction in ST-elevation myocardial infarction5, we hypothesised that early short-term ET receptor antagonism improves microvascular function after primary PCI in human ST-elevation acute coronary syndrome (STE-ACS).
Magnetic resonance imaging (MRI) is a sensitive tool to detect and quantify microvascular reperfusion in AMI14 and stable coronary artery disease15. Delayed first-pass perfusion is associated with impaired recovery of ventricular function after AMI14. Moreover, microcirculatory dysfunction detected by MRI has been suggested to be a stronger predictor of cardiac remodelling than Thrombolysis In Myocardial Infarction (TIMI) coronary flow, myocardial blush grade, ST-segment resolution and infarct size16.
The aim of this single-centre, randomised, placebo-controlled, double-blind pilot study was to investigate the safety and efficacy of the ETA receptor antagonist BQ-123 in STE-ACS.
Methods
Patients and inclusion criteria
Between May 2007 and August 2009, 117 men or post-menopausal women aged 18 years and above with posterior wall STE-ACS, defined as ischaemic chest pain for >30 minutes within <12 hours and new ST-segment elevation in two or more contiguous electrocardiographic leads or in case of a true posterior infarction reciprocal ST-segment depressions in V1 and V2 >1 mm, were screened for study eligibility. Important exclusion criteria were cardiogenic shock (systolic blood pressure <90 mmHg or need for inotropic support); thrombolytic therapy; history of myocardial infarction; history of congestive heart failure; metal implants contraindicating MRI; inability to read, understand and sign the informed consent; and participation in another clinical study. TIMI grade 0 or 1 coronary flow in the infarct-related coronary artery (IRA) with planned primary PCI of the culprit lesion were angiographic inclusion criteria. All patients received unfractionated heparin to reach an activated clotting time of 300 seconds, 500 mg of acetylsalicylic acid and 600 mg of clopidogrel prior to PCI. Patients experiencing angiographic no-reflow received intracoronary adenosine and nitrate. The study was approved by the ethics committee of the Medical University of Vienna and the Austrian Federal Office for Safety in Health Care and was conducted in accordance with the Declaration of Helsinki. Patients were included after providing written informed consent.
Study protocol
Treatment and randomisation
Operator-blinded treatment allocation was performed with computer-generated 1:1 allotment using numbered envelopes, which were opened by study personnel after informed consent had been obtained, and TIMI grade 0/1 flow in the IRA had been documented. Patients were randomised to receive BQ-123 (Clinalfa, Läufelfingen, Switzerland) dissolved in 50 mL of 0.9% sodium chloride (NaCl) at 400 nmol/minute intravenously or placebo (NaCl) over 60 minutes, immediately before guidewire insertion17. BQ-123 induces potent inhibition of [125I]ET-1 binding to ETA receptors (IC50: 7.3 nM) on vascular smooth muscle cells, but barely inhibits binding to ETB receptors (IC50: 18 microM)18. Concomitant medical therapy was given according to standard of care; the use of thrombectomy devices and glycoprotein IIb/IIIa antagonist were at the operator’s discretion.
Magnetic resonance imaging (MRI)
MRI was performed six days and six months after PCI on a clinical 1.5 Tesla MRI scanner (Siemens Avanto, Erlangen, Germany). Cine imaging for the assessment of left ventricular end systolic volume (LVESV), left ventricular end diastolic volume (LVEDV) and left ventricular ejection fraction (LVEF) was performed using a steady-state free precession pulse sequence applying standard protocols. A cumulative dose of 0.15 mmol/kg of gadolinium-DTPA (dimeglumine gadopentetate; Magnevist®; Bayer Schering Pharma AG, Berlin, Germany) was administered with a power injector. Microvascular perfusion was graded in a blinded fashion at rest using first-pass perfusion images (flash perfusion, TR/TE 178.34/1.02, flip angle 15°, slice thickness 10 mm).
The time to 50% maximal myocardial enhancement (T50%max) in the most severely affected myocardial segment subtended by the IRA was calculated and compared with a remote reference segment14. Delays were corrected for perfusion in normal areas in each individual patient. Delayed enhancement was evaluated 10 minutes after contrast administration permitting the expression of infarct size in per cent of total left ventricular mass19. Areas of hypo-enhancement within infarcted myocardium were defined as areas of “no-reflow” and were added to the infarct size. Myocardial salvage index was calculated as myocardial area at risk as determined by T2 weighted imaging minus infarct size on T1 weighted imaging following contrast administration divided by the area at risk20 using CMR42 version 3.2 (Circle Cardiovascular Imaging Inc, Calgary, Canada).
Other outcome parameters
Serum creatine phosphokinase (CK) levels were measured at 6-12, 48 and 72 hours after PCI. A clinical follow-up was performed 30 days after PCI including a clinical examination, ECG, blood pressure measurement, routine laboratory testing including liver enzymes and amino-terminal pro-B-type natriuretic peptide (NT-proBNP) measurement. Major adverse cardiac events (MACE, cardiovascular death, re-hospitalisation for unstable angina and AMI, or hospitalisation for worsening heart failure), and severe adverse events were recorded.
Outcome variables
The pre-specified primary efficacy endpoint was myocardial perfusion delay determined by MRI (T50%max). Final TIMI flow, myocardial blush grade, LVEF, final infarct size and myocardial salvage index by MRI, NT-proBNP, enzymatic infarct size, ST-segment resolution, plasma liver enzyme levels and 30-day MACE were secondary endpoints.
Statistical analysis
This study was a pilot safety study and the sample size was comparable with other recent pilot-trials in similar settings21. The sample size of 22 in each group had a 90% power to detect a difference in means of one standard deviation using a two group t-test with 0.05 two-sided significance level. Fifty-seven patients were included in order to account for drop-outs and technical difficulties. Data were analysed on an intention-to-treat basis. Results were expressed as medians (25th-75th percentiles). Two independent observers who were blinded to each others’ findings assessed T50%max off-line. Limits of agreement were ±2.99 (intra-observer) and ±2.25 (inter-observer). Comparisons between groups were made using the unpaired t-test. The non-parametric Mann-Whitney U test was used in case of outliers or asymmetric distributions. The chi-squared test was used to compare frequencies. An analysis of covariance (ANCOVA) model was performed in order to adjust for the time between PCI and MRI assessment, which was imputed as the log-transformed number of days after PCI. A p-value of <0.05 was considered significant. The study was monitored by the Academic Studies Support Office of the Medical University of Vienna.
Role of the funding source
The funding sources had no role in the study design, data collection, data analysis, data interpretation or writing of the report.
Results
Study population
Patient characteristics are shown in Table 1. Of 57 randomised patients, 28 were assigned to receive BQ-123 and 29 were assigned to placebo. Patient screening, enrolment, randomisation, and follow-up are shown in Figure 1. Three protocol violations occurred in patients who were included in the study despite an onset of pain more than 12 hours before. All three were in the placebo arm.
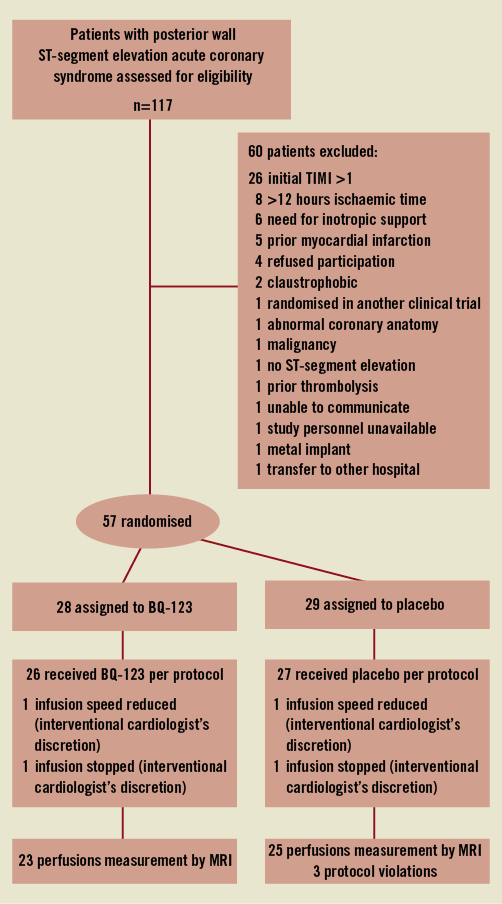
Figure 1. Screening, enrolment, randomisation, and follow-up of study patients. TIMI: Thrombolysis In Myocardial Infarction; MRI: magnetic resonance imaging
The median (25th–75th percentile) age was 59 years (52-69); 11 (20%) patients were female. Ischaemic time from onset of chest pain to first balloon inflation or thrombectomy was four hours (2-6), and the time from first medical contact to balloon22 was 90 minutes (71-110). Four (14%) of the patients randomised to BQ-123 and seven (27%) of the patients randomised to placebo were in KILLIP classes ≥2 (p=0.249). Comorbidities were equally distributed between groups. Except for high-density lipoprotein cholesterol and body mass index no differences in baseline clinical characteristics and concomitant medications were found between the treatment group and the control group.
Primary endpoint
At 6.0 days (4-11.5) after PCI, shorter microvessel perfusion delays (T50%max) were observed in patients randomised to receive BQ-123 than in patients randomised to placebo (1.8 sec [0.7-3.4] versus 3.3 sec [2.3-5.4], p=0.005; Figure 2). This difference remained significant after correcting for the time interval between PCI and MRI (p=0.006). There was no impact of timing of the MRI examination on the primary endpoint (p=0.95).
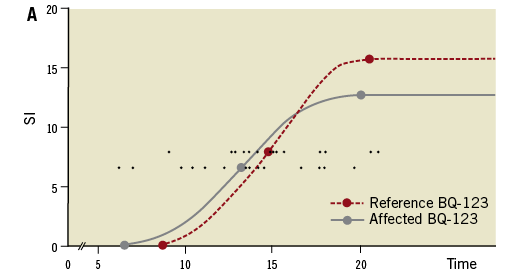
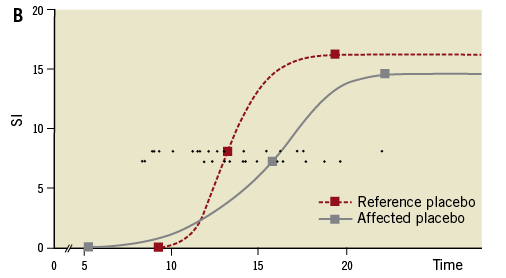
Figure 2. Myocardial contrast media wash-in. Closed lines represent media wash-in to the ischaemic myocardial territory subtended by the infarct-related artery. Dashed lines depict flow to remote non-ischaemic myocardial territory. Round (representing BQ-123 treated patients) and square (representing placebo-treated patients) symbols show the mean time from intravenous contrast media injection to the onset, to 50% of the maximal enhancement (T50%max), and to the peak of microvascular wash-in. For the primary endpoint the time difference in myocardial contrast wash-in between the most affected myocardial segment and an unaffected reference segment was assessed. Delays were therefore corrected for perfusion in normal areas in each individual patient. (T50%max, each patient is represented by a small round symbol on both horizontal lines). SI: signal intensity
No side branch occlusions or bleeding complications occurred. In 17 individuals, post-dilation of the implanted stent was necessary. None of these patients experienced angiographic no-reflow. Furthermore, there was no statistical difference in ST-segment resolution (BQ-123 group: 88% [50-100]; control group: 80% [60-100], p=0.845) one hour after the intervention.
At the end of the procedure, 52 patients (96%) had a TIMI 3 coronary flow. All patients of the BQ-123 treated group presented with a final TIMI 3 coronary flow compared with 24 patients (92%) in the placebo-treated group (Table 1). Myocardial blush grades were not significantly different between the groups (p=0.336, Table 1).
At 10 hours (6-15) after first medical contact, maximum CK levels were 1,365 U/L (766-2,139) in BQ-123 treated patients and 2,132 U/L (1,531-2,735) in the placebo group (p=0.014, Figure 3). Peak levels of troponin T were 3.3 U/L (2.3-5.8) in BQ-123 treated patients and 5.3 U/L (3.3-7.8) in the placebo group (p=0.046).
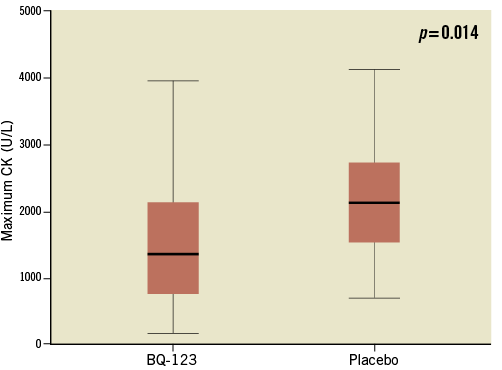
Figure 3. Box plots representing peak creatine phosphokinase levels in BQ-123-treated and in placebo-treated patients (p=0.014). Respective levels were reached 10 hours (6-15) after first medical contact. CK: creatine phosphokinase
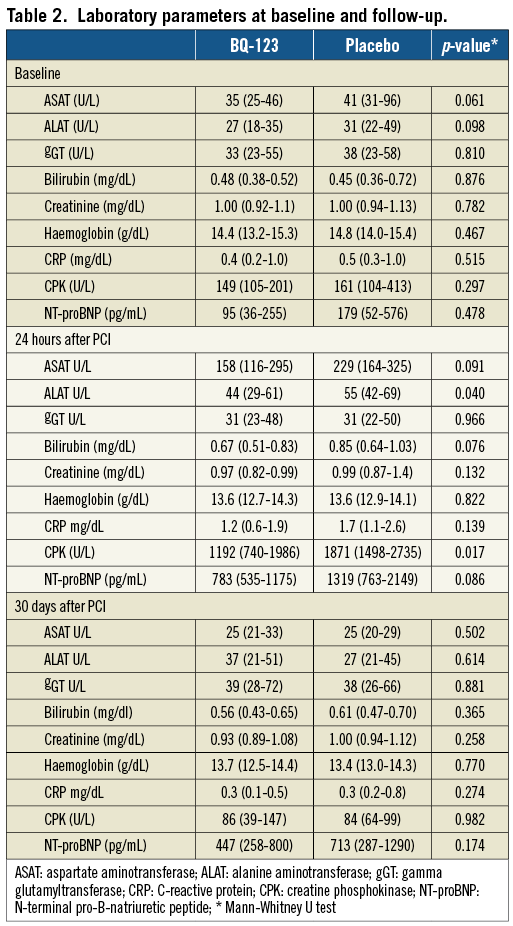
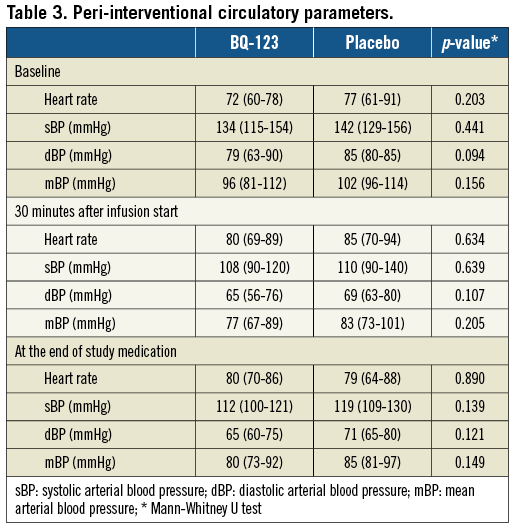
Plasma liver enzyme levels, C-reactive protein, creatine phosphokinase, and amino-terminal pro-B natriuretic peptide levels at baseline, 24 hours and 30 days after PCI are presented in Table 2. No haemodynamic compromise was observed in association with the drug application (Table 3).
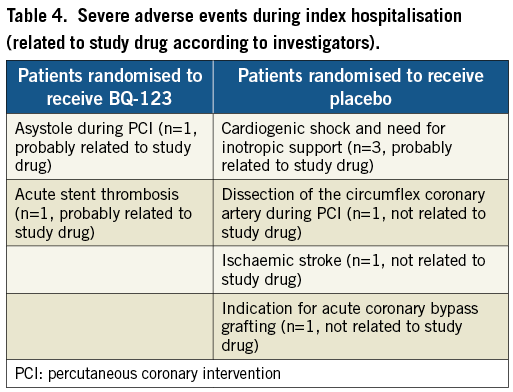
Severe adverse events during the index hospitalisation are presented in Table 4. Within 30 days of follow-up no MACE occurred after discharge.
At discharge, 26 patients (93%) randomised to BQ-123 were on a beta-blocker, compared to 24 (93%) in the placebo group (p=1.000). Twenty-six (93%, BQ-123) and 23 (86%, placebo group) received an angiotensin converting enzyme inhibitor or angiotensin receptor blocker (p=0.663). Twenty-six (93%, BQ-123) and 26 (100%, placebo group) were on statins (p=0.491). All patients were on 100 mg acetylsalicylic acid and clopidogrel at discharge.
At 30-day follow-up, 24 patients (86%) of the BQ-123 group were in New York Health Association (NYHA) class I and four (14%) in NYHA class II, whereas in the placebo group 18 patients (69%) were in NYHA class I, four (15%) in NYHA class II and four (15%) in NYHA class III (p=0.091). Furthermore, 27 patients (96%) in the BQ-123 group reported Canadian Cardiovascular Society (CCS) class I angina and one (4%) CCS class III angina. Of the placebo-treated patients 23 (89%) reported CCS class I angina and three (12%) reported CCS class II angina (p=0.342).
Secondary endpoints assessed with MRI are displayed in Table 5.
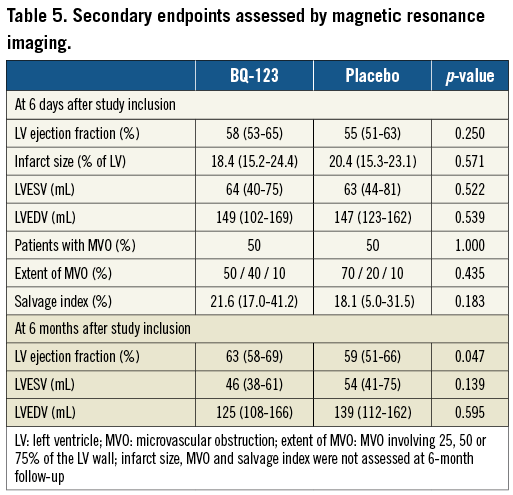
At six months, all patients were alive, and microvascular perfusion delays measured at index hospitalisation were inversely correlated with LVEF at six months (R=-0.313, p=0.047) (Figure 4).
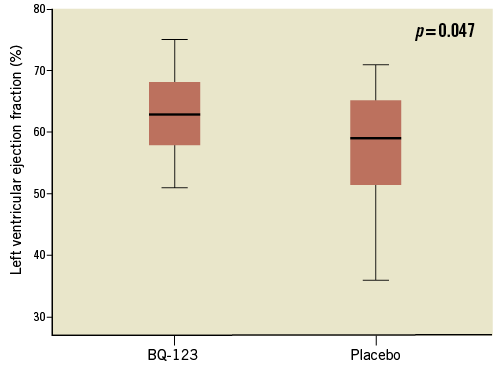
Figure 4. Box plots representing MRI left ventricular ejection fraction of patients randomised to BQ-123, and placebo-treated patients, at six months.
Discussion
This is the first trial evaluating the effects of ERA in patients with STE-ACS undergoing primary PCI within a well-established network23. Although animal models using ERA in AMI differ with regard to duration of ET receptor blockade24, the present pilot study suggests that short-term administration of the selective ETA receptor blocker BQ-123 in patients with STE-ACS is safe and associated with a biological signal of improved myocardial tissue level perfusion and improved LVEF at six months. This effect was observed in the presence of optimal medical therapy, frequent utilisation of thrombectomy, and guideline-adherent first-medical-contact to balloon times (Table 1). An extremely high rate of >70% of ST-resolution occurred in this trial. The Vienna infarct network23 has improved further over recent years, with ST resolution rates exceeding those of other published series (57%25 and 63% of patients with >70% resolution26). This explains why no differences in one-hour ST-segment resolution rates were detectable between BQ-123 and placebo-treated patients in our trial.
Our study population is more homogenous than the patients reported by Taylor and colleagues who studied microvascular reperfusion after infarct angioplasty with magnetic resonance imaging14. Taylor et al14 included patients with TIMI grade 0 to 2 flow in the IRA (anterior and posterior infarcts). By contrast, the majority of our patients presented with TIMI grade 0 coronary flow in the initial angiogram. This explains severer microvascular perfusion delays in our study. Additional differences may result from timing. Microvascular perfusion measurements at 6.0 days (4-11.5) instead of 24 hours14 was preferred because of an alleged improved prognostic value of microvascular perfusion at several days after PCI27. Furthermore, recent work illustrates that myocardial injury remains stable for over a week, providing a window for evaluation by MRI28. The calculation of microvascular perfusion delay is based on the difference of a single patient’s affected against his/her non-affected myocardial perfusion delay, the readouts of which have been displayed within a single panel (Figure 2).
Although ischaemia/reperfusion injury is multi-factorial, including oxidative stress, intracellular calcium overload, rapid pH changes, neutrophil accumulation29, and apoptosis, ET plays a key role24,30,31. In ischaemia/reperfusion injury, ET mediates coronary microvascular constriction via the ETA receptor32, enhances neutrophil adhesion33, boosts reactive oxygen species generation34, and furthers hypoxia-induced apoptosis of cardiomyocytes35. In addition, ET affects cardiac fibroblast proliferation36, modulates the mitochondrial permeability transition pore which impacts cardiomyocyte hypertrophy and post-infarction remodeling37. Our group has recently investigated the in vitro vasoconstrictive effect of fresh human coronary thrombi in a porcine coronary artery ring model. We could show that pre-incubation of vascular rings with an ET receptor antagonist leads to significant attenuation of the vasoconstrictive response to human acute coronary thrombus homogenates5. Inhibition of ET-dependent neutrophil activation may represent a potential explanation for the benefit observed in addition to vasodilation38. Although it has been suggested that ETB receptors play a role in the clearance of ET-1 in man and that their blockade may be deleterious for patients with heart failure39, the evidence for pharmacological interference on the ETB receptor is not supported by clinical data40.
BQ-123 dosing was based on the observation that intravenous applications of BQ-123 above 300 nmol/minute had reproducible systemic effects and that dosages of 1,000 nmol/minute lowered systemic blood pressure41. Infusion of BQ-123 for 15 minutes produced haemodynamic effects for up to four hours, even though BQ-123 plasma concentrations fell to 10% by 30 minutes after the infusion, indicating a sustained pharmacodynamic effect beyond the termination of continuous intravenous application. It may be speculated that the effect of BQ-123 covers a critical period in the natural history of AMI because systemic ET levels usually return to normal values within 24 hours42. Furthermore, acute short-term administration may prevent acute detrimental effects of ET in AMI without compromising subsequent myocardial repair24. The initiation of an ET receptor blockade prior to mechanical reperfusion may be essential, because mechanical disruption of semi-solid acute coronary thrombus5 may quickly liberate large amounts of ET causing sustained coronary vasoconstriction43,44 especially in the presence of cytokines from other sources45.
STE-ACS patients with initial TIMI grade 0 or 1 flow represent a high-risk population46. Peak CK levels were comparable with other studies in ST-elevation myocardial infarction in that peak total CK levels were 1,420 U/mL47 or 922 mg/dL and 1,973 mg/dL48. Infarct sizes assessed by MRI (18.4% and 20.4%) were similar to those of other trials (16.9%16, 17.1%49 and 15.0%50). Our study demonstrates that ETA receptor blockade in STE-ACS is safe. We observed no elevation of transaminases (Table 2) and no haemodynamic compromise (Table 3). Data on microvascular perfusion should be interpreted with caution, because of variable flow (T50%max) in the reference regions illustrating relatively large individual variation. BQ-123 may improve tissue level perfusion assessed by cardiac MRI (Figure 2), and reduce enzymatic infarct size (Figure 3). Tissue level perfusion is an important predictor of clinical outcome51, and the actual amount of microvascular improvement translated into differences52 in LVEF at six months after the event (Table 5).
Limitations
The study is limited by its “proof-of-concept” nature: small size, single centre design, bias towards posterior wall infarctions, short observation time, and surrogate endpoints. Since no baseline measurements were performed before application of the investigational drug, it cannot be excluded that the observed differences were due to baseline differences. However, this proof-of-concept study was stimulated by contradictory animal studies12, and naturally requires a smaller sample size than outcome studies. The sample size of the present study is comparable with others performed in similar settings21. In addition, modern infarct treatment has led to excellent ST-segment resolutions and outcomes in the placebo group, which accounts for the lack of difference in ST-segment resolution and infarct size by delayed contrast hyper enhancement. However, those were not primary endpoints.
The study was limited to randomise posterior infarcts because of data suggesting adverse cardiac remodelling after ET receptor blockade in anterior infarctions12. Although greater changes in left ventricular volumes are to be expected in anterior than in posterior myocardial infarctions53, scar size has been the strongest independent predictor of LV volumes, independent of scar localisation54. Because of the small sample size, no correction for potential confounders was undertaken.
Conclusion
Short-term administration of a selective ETA receptor blocker during primary PCI in patients with STE-ACS is safe and may improve tissue-level perfusion, translating into a minute improvement of LVEF at six months. The data warrant a larger clinical trial with longer exposure to ERA.
Acknowledgments
The authors thank Veronika Seidl for technical assistance.
Funding
The study was supported by the Austrian National Bank’s “Jubilaeumsfonds” to C. Adlbrecht #12758, the Austrian Society of Cardiology to C. Adlbrecht (2005 and 2009) and the Hans und Blanca Moser Stiftung to B. Redwan (2007).
Conflict of interest statement
The authors have no conflict of interest to declare.