
Introduction
Stenting procedures for bifurcation lesions can be complex yet are often necessary with percutaneous coronary interventions. There are few resources and clinically applicable models for practitioners to visualise stenting techniques1. Optical coherence tomography (OCT) and intravascular ultrasound (IVUS) are beneficial for real-time procedural visualisation; however, reconstructions of the left main are difficult with OCT and often IVUS produces artefacts2. Evaluations of implantable cardiac devices require innovative and critical testing during the design process and for understanding procedural outcomes. Other benchtop models for bifurcation stenting are valuable3,4; however, Visible Heart® methodologies offer the unique capability of demonstrating these procedures within physiologic anatomy with analogous compliance and flow to human anatomy5.
Here we describe direct endoscopic visualisation of a coronary bifurcation stenting procedure in a human heart with subclinical coronary disease. Multimodal visualisations provide critical stepwise education for patients, clinicians, and device design engineers, as well as insights relative to the device-tissue interface within a human heart (Figure 1, Figure 2, Moving image 1).
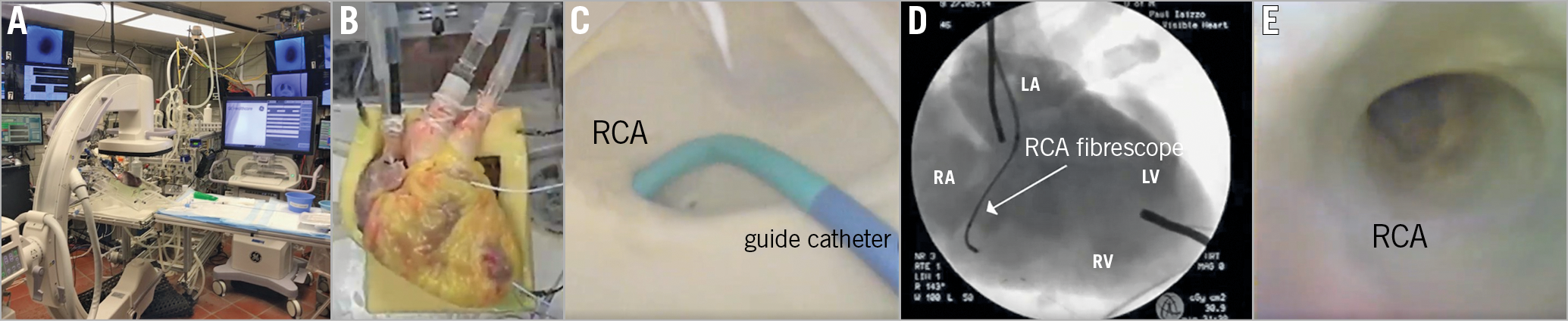
Figure 1. Visible Heart methodologies. A) C-arm, back table, and Visible Heart apparatus and monitoring capabilities. B) Reanimated human heart from a 78-year-old female donor. C) Guide catheter placed in the right coronary artery (RCA). D) 2.4 mm fibrescope placed in the RCA. E) Direct visualisation of the RCA. LA: left atrium; LV: left ventricle; RA: right atrium; RV: right ventricle
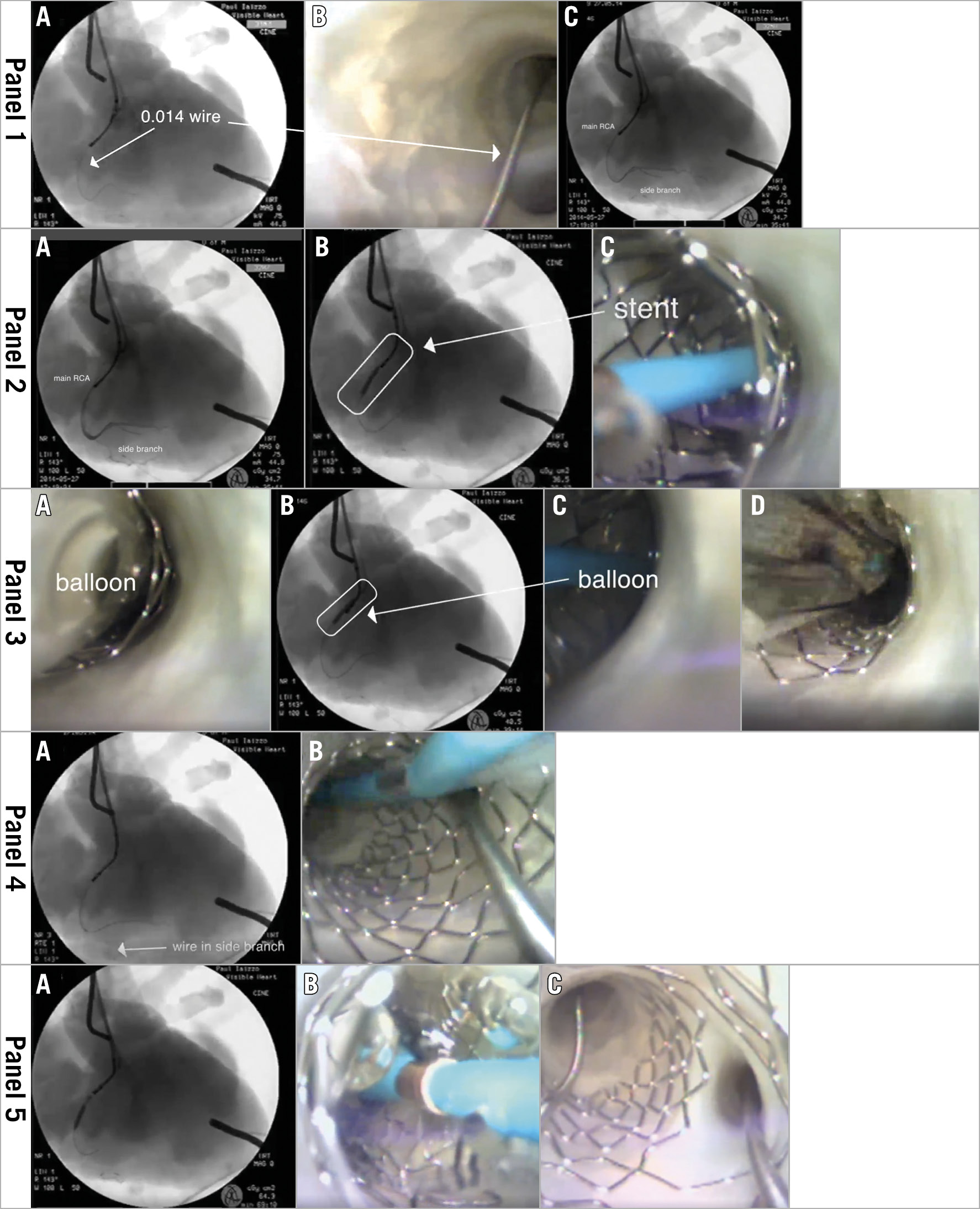
Figure 2. Steps of the provisional stenting technique. Panel 1. A) Fluoroscopic image of guide catheter positioned in right coronary artery (RCA) and placement of a 0.014-inch wire in the main branch. B) Direct visualisation of the target bifurcation and a 0.014-inch wire within the main branch. C) Fluoroscopic image of the main and side branches wired. Panel 2. A) Fluoroscopic image of contrast injection via the guide catheter in the RCA. B) Fluoroscopic guidance of stent deployment within the main RCA branch. C) Direct visualisation of stent deployment in the main branch vessel. Panel 3. A) Short balloon (sized 1:1) positioned to cover the proximal edge of the main branch stent; one can visualise malapposition of stent struts to the vessel wall. B) Balloon inflation under fluoroscopy. C) Balloon expanding the stent within the main branch. D) Direct visualisation of balloon deflation and properly apposed stent struts onto the vessel wall. Panel 4. A) Fluoroscopic image of the wire advanced into the side branch. B) Direct visualisation of the wire through the distal strut of the main branch stent covering the side branch ostia. Panel 5. A) Fluoroscopic image obtained during kissing balloon dilation. B) Direct visualisation of the kissing balloon technique. C) Direct visualisation of the final result of the provisional stenting technique (result of final proximal optimisation technique not shown).
Methods
A heart, not viable for transplantation, was procured from a 78-year-old female organ donor via LifeSource (St. Paul, MN, USA). Research was approved by the University of Minnesota’s Institutional Review Board and LifeSource’s research board, and consent from the donor’s family was obtained. Upon reanimation with a clear modified Krebs-Henseleit buffer, according to previously described methodologies5, the heart elicited an intrinsic normal rhythm that was sustained with viable haemodynamic function. The right coronary artery (RCA) was accessed with a guide catheter and visualised (Figure 1A-Figure 1E). Images were simultaneously captured with endoscopic footage from the ascending aorta and right coronary artery via 4.0 mm and 2.4 mm videoscopes (IPLEX FX; Olympus, Tokyo, Japan) and fluoroscopy.
Results
Endovascular visualisations are paired with fluoroscopic imaging to describe steps of a provisional stenting technique within a human’s coronary anatomy.
WIRING SIDE AND MAIN BRANCHES
The main branch was wired with a 0.014-inch Cougar™ guidewire (Medtronic, Minneapolis, MN, USA) and the side branch (small acute marginal branch) was also wired, as seen under fluoroscopy and direct visualisation with the 2.4 mm videoscope (Figure 2, Panel 1A-1C). A stenotic lesion location was not identified in this heart; however, a “typical” location where a provisional stenting technique is identified with visible vessel calcification is shown in Figure 2, Panel 2A-2B. This location was utilised for demonstrating the stenting procedure; the main vessel just proximal to this bifurcation (crux at the RCA) elicited multiple plaques and fatty streaks, and minor calcified nodules.
CONTRAST BOLUS AND MAIN BRANCH STENT DEPLOYMENT
A contrast bolus was delivered through the guide catheter and stenting was sized to the main branch utilising estimation under fluoroscopy. The Integrity™ stent (Medtronic) was deployed with 16 atm of balloon pressure to ~3.5 mm (Figure 2, Panel 2A-2C).
PROXIMAL OPTIMISATION TECHNIQUE
The proximal optimisation technique (POT) was performed on the proximal end of the stent with a short non-compliant balloon, sized 1:1 according to the proximal main branch size (Figure 2, Panel 3A-3D). This technique ensured proper apposition of the stent to the vessel wall. Consensus data suggest that this reduces the risk of the stent overlying the side branch or disrupting the neocarina1.
WIRING THE SIDE BRANCH THROUGH THE MAIN BRANCH STENT
To gain access to the side branch, a wire was crossed through the main branch struts (Figure 2, Panel 4A-4B). It is important to aim for one of the distal stent struts that covers the side branch ostia.
FINAL KISSING BALLOON TECHNIQUE AND VISUALISATION OF THE NEOCARINA FORMED
A second balloon was placed over the side branch wire; the main and side branch balloons were inflated simultaneously (10 atm each) to perform the kissing balloon technique (KBT) (Figure 2, Panel 5A-5C). This produces an optimally apposed main branch stent while opening struts to generate a neocarina at the ostia of the side branch. The overall technique was finalised by performing POT of the main branch stent - inflating a short non-compliant balloon in the main branch stent proximal to the bifurcation area in order to ensure proper stent apposition and correct proximal potential or changed geometry (bottleneck) in the proximal stent segment after potential KBT misalignment.
Discussion
Visible Heart methodologies have enabled our laboratory to reanimate 85 human hearts and directly observe the critical steps involved in complex bifurcation stenting procedures, offering valuable insights on the dynamics and effects of different procedural techniques. This approach is closer to native human physiology and/or pathophysiology than utilising silicone tubes or other bench testing techniques due to dynamic compliance, flow, and capacity for visualisation within real anatomies.
Limitations
The stenting procedure was performed in a reanimated human heart with minimal coronary artery disease, and thus is considered an idealised outcome.
Conclusion
We provide educational still and moving images of a provisional stenting technique in a reanimated human heart.
|
Impact on daily practice Our unique ability to reanimate human hearts offers educational visualisation of bifurcation procedures that is valuable for patients, clinicians, and device design engineers. |
Acknowledgements
We thank LifeSource, the donor, and her family for their generous donation.
Funding
This research was funded by the Institute for Engineering in Medicine and a Medtronic research contract.
Conflict of interest statement
P. Iaizzo has a research contract with Medtronic. F. Burzotta has received speaker fees from Medtronic, Abbott, and Abiomed. J.F. Lassen has received speaker fees from Medtronic, Abbott, Biosensors, and Boston Scientific. T.L. Iles has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Provisional bifurcation stenting procedure.

