Abstract
John Doe, an 81-year-old patient with a significant distal left main (LM) stenosis, was treated using a provisional stenting approach. As part of an European Bifurcation Club (EBC) project, the complete stenting procedure was repeated using computational modelling. First, a tailored three-dimensional (3D) reconstruction of the bifurcation anatomy was created by fusion of multislice computed tomography (CT) imaging and intravascular ultrasound. Second, finite element analysis was employed to deploy and post-dilate the stent virtually within the generated patient-specific anatomical bifurcation model. Finally, blood flow was modelled using computational fluid dynamics. This proof-of-concept study demonstrated the feasibility of such patient-specific simulations for bifurcation stenting and has provided unique insights into the bifurcation anatomy, the technical aspects of LM bifurcation stenting, and the positive impact of adequate post-dilatation on blood flow patterns. Potential clinical applications such as virtual trials and preoperative planning seem feasible but require a thorough clinical validation of the predictive power of these computer simulations.
Introduction
This paper is specifically dedicated to patient-specific virtual simulation of coronary bifurcation stenting. A proof of concept is reported and a number of potential applications are discussed. For example, the 3D anatomical models created for virtual simulation can offer the operator an angiographic target for visualising complex anatomy and even offer an intraluminal insight. Furthermore, these virtual models may also be used to compare the subtleties of various coronary bifurcation stenting techniques for research purposes, for teaching the cardiologist, or in order to identify the optimal strategy for each individual anatomy.
The concept of virtual stent deployment in a coronary bifurcation for studying treatment of bifurcation lesions was first introduced at the European Bifurcation Club Meeting in 2011. The conventional angiography-guided intervention and the virtual simulations of the patient “John Doe” were both presented. In the case of the virtual simulation, exactly the same technique and materials were employed.
John Doe is an 81-year-old patient who presented with breathlessness on exertion. Angiography demonstrated severe triple-vessel disease with a distal left main stenosis (fractional flow reserve [FFR] of 0.64 in the left anterior descending artery [LAD] and 0.72 in the circumflex), moderate ostial LAD lesion, a long lesion of the second marginal and a significant mid right coronary artery (RCA) stenosis. The virtual stent deployment simulations focused on the treatment of the distal left main stenosis.
In order to generate the virtual 3D lumen model, CT angiography and IVUS pullbacks of both the LAD and circumflex were performed. Firstly the RCA (XIENCE PRIME 2.5×28 mm; Abbott Vascular, Santa Clara, CA, USA) and marginal (XIENCE PRIME 2.5×23 mm and 2.25×12 mm) branches were treated. Figure 1 demonstrates the LM percutaneous coronary intervention. One year after the procedure the patient was asymptomatic with a normal stress MRI.
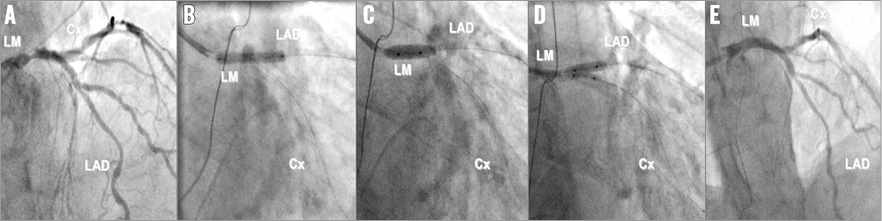
Figure 1. John Doe stenting. A) Distal LM stenosis. B) Stenting from LM to LAD adapted to LAD diameter (3.0×23 mm XIENCE PRIME). C) Proximal optimisation technique (POT) with a balloon adapted to LM (4.5 mm non-compliant). D) Kissing balloon with diameters based on distal segments (3.5 mm for LAD and 2.5 mm for LCX). E) Final result.
From patient imaging to 3D bifurcation model
Several approaches with different levels of accuracy and complexity can be adopted when aiming at 3D reconstruction of the bifurcation region, including the wall and vessel components. The classic method to image the lumen of coronary arteries is (biplane) angiography using two different X-ray views, ideally under an angle of 90 but not less than 30 degrees1. Currently, some commercial software packages allow 3D reconstruction of the coronary arteries including the bifurcation region based on angiography2,3. Since only two views are used, the exact location and shape of the bifurcation region are a challenge. With carefully chosen views one might be able to capture the take-off of the side branch correctly1, but the overall 3D geometry of the bifurcation region still needs improvement, and the need for further improvement largely depends on its application.
Multislice CT is an emerging non-invasive imaging modality which generates a 3D voxel space of the coronary bifurcation. Even though this technique relies on a combination of multiple X-ray views and the number of detectors is growing, its final resolution is currently still too low (0.25-0.625 mm) to obtain accurate 3D reconstructions of the bifurcation region, including precise wall information. The resolution of intravascular imaging techniques is much higher. For instance, intravascular ultrasound and optical coherence tomography have an in-plane resolution of 0.15 mm and 0.015 mm, respectively, with which it is possible to image even small structures and composition inside the vessel wall, including the lipid pool and fibrous cap thickness. However, intravascular ultrasound imaging is hampered when the plaque is heavily calcified, not allowing visualisation of the wall behind such structures. Although these intravascular imaging techniques have their own advantages and disadvantages with respect to visualising certain plaque features or deep structures, the lumen shape can be accurately assessed in the majority of cases. However, all these techniques lack the information of the 3D shape of the artery, and therefore fusion of non/less invasive imaging techniques with intravascular imaging is a good alternative when considering 3D reconstruction of the bifurcation region of the coronary artery4,5. Registration of CT and intravascular imaging techniques depends on local landmarks, which are often abundantly present in the bifurcation region. Although registration of intravascular imaging with CT inherently houses inaccuracies, it is the best option today for obtaining a 3D reconstruction of the lumen and vessel wall of the bifurcation region. For the John Doe case, CT was combined with intravascular ultrasound in both branches to create a virtual model of the LM bifurcation (Figure 2).
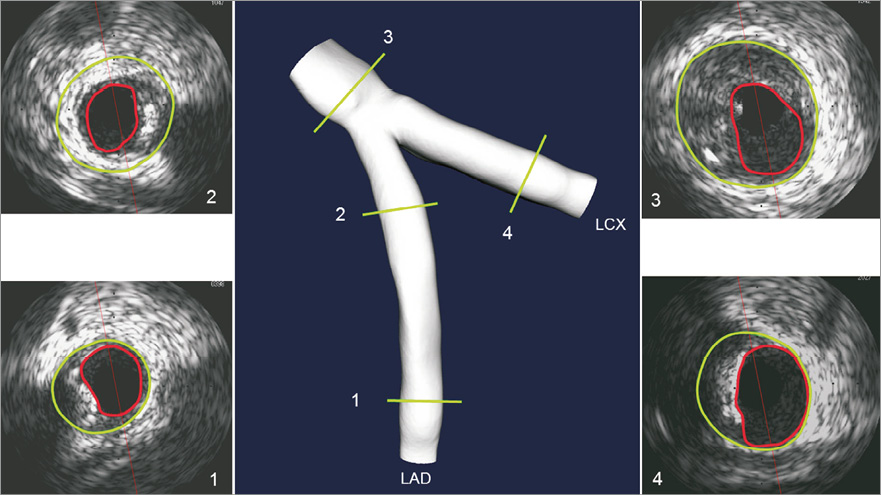
Figure 2. John Doe case. 3D reconstruction of the bifurcation region including the side branch using MSCT and intravascular ultrasound in both branches showing the cross-sections at several locations.
Virtual bifurcation stenting
A computer modelling technique called finite element analysis makes it possible to perform and evaluate different bifurcation stenting strategies virtually, using the patient-specific LM model. A first step in the simulation process is to generate stent and balloon models that are accurate in terms of geometry and mechanical behaviour (e.g., compliance). Then, the different steps of a bifurcation stenting procedure can be modelled and stent deformations after each step can be assessed and quantified. Furthermore, stress within the arterial wall can be investigated. The accuracy of this technique has been verified previously by comparing the results of virtual bifurcation stenting with in vitro bench testing6. Detailed information on the methodology has been described by Mortier et al7.
Similar to the real treatment of John Doe, a 3.0×23 mm XIENCE PRIME was deployed virtually at 12 atm, followed by the proximal optimisation technique (POT) (4.5×10 mm NC Hiryu® balloon; Terumo Corp., Tokyo, Japan, 14 atm) and final kissing inflation (3.5×10 mm NC Hiryu in LAD, 2.5×10 mm NC Hiryu in LCX, 14 atm). Resulting stent deformations after stent deployment and POT are depicted in Figure 3. An interesting aspect of (patient-specific) virtual bifurcation stenting is that multiple alternative treatment approaches can be compared. As an example, the impact of a different stent sizing strategy was investigated by deploying a 3.5 mm XIENCE PRIME in the John Doe model (Figure 4).
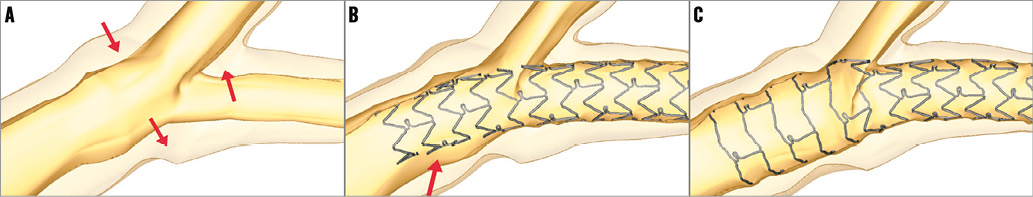
Figure 3. Virtual stent deployment and POT. By comparing the bifurcation model before (A) and after stent deployment (B), it can be noticed that there is a reduction of the side branch (LCX) diameter. This reduction in diameter is the result of two mechanisms: 1) carina shift (upward arrow in panel A) and 2) a downward movement of the lateral walls due to straightening of the bifurcation (two downward arrows in panel A). POT (C) further decreases the SB diameter. After stent deployment, a considerable malapposition in the LM can be observed (arrow in panel B), which is completely resolved after POT (C).
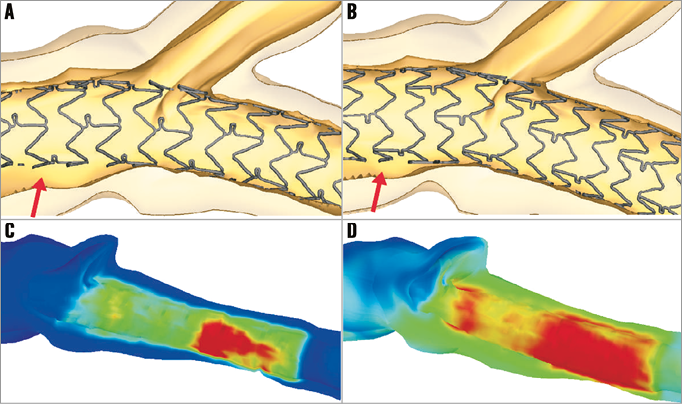
Figure 4. Illustration of the impact of stent size selection by patient-specific virtual bifurcation stenting. The stent size for John Doe was selected based on the distal (LAD) branch diameter and a 3.0 mm XIENCE PRIME was implanted (A). Selecting a larger stent size (B) reduces the amount of malapposition in the LM after the initial stent deployment (arrows), but increases carina shift. Furthermore, a larger stent size results in higher strains within the vessel wall ([C] 3.0 mm stent; [D] 3.5 mm stent; red shows region with high vessel wall strain), which may increase the risk of a distal dissection.
Flow modelling
The final geometries obtained through the mechanical simulations were used as the fluid domains to perform transient fluid dynamic analyses8. At the inlet cross-section a pulsatile flow tracing with a plug velocity profile was applied, which is representative of a human left main coronary artery (Figure 5)9. At the outlets the flow distribution was calculated using the relations defined by van der Giessen10, based on human coronary bifurcation data. The following flow splits were imposed on both models: 55% for the main branch and 45% for the side branch. The arterial wall and the stent were assumed to be rigid. The fluid dynamic simulations were carried out using the commercial software ANSYS Fluent (ANSYS Inc., Canonsburg, PA, USA). Detailed information on the methods and the limitations of computational flow modelling are described elsewhere11,12.
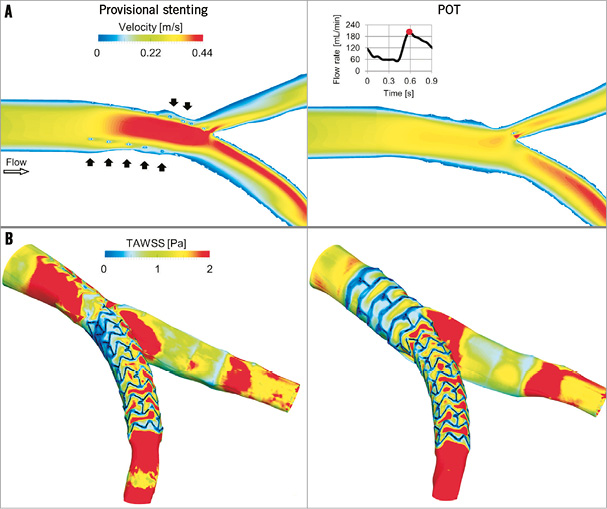
Figure 5. Fluid dynamic results of the provisional stenting (left) and POT (right) models. A) Velocity contour maps on an internal plane of the bifurcation at peak flow rate. In the provisional stenting, where the malapposed struts are clearly visible (black arrows), higher velocities are present as the stent behaves as a cylindrical hurdle in the flow stream. Indeed, in the POT, struts are well apposed to the wall and do not obstruct the blood stream. B) Time-averaged WSS (TAWSS) contour maps. Low WSS are generated next to the stent struts. A wide area with low WSS is present in the bifurcation region in the provisional stenting model and in the proximal part of the stent in the POT model.
The velocity patterns inside the stented vessel and the wall shear stress (WSS) along the arterial wall are shown after stent deployment and POT in Figure 5. Moving image 1 shows the fluid dynamics in terms of animated pathlines. These are haemodynamic quantities that influence flow-related processes leading to restenosis. Indeed, low WSS and disturbed fluid dynamics have been observed in areas where biological compounds are active13. Furthermore, in vivo studies on stented arteries showed that low WSS are associated with neointimal hyperplasia14,15.
Current and future applications
With this proof-of-concept study, the EBC has demonstrated the feasibility of performing patient-specific computer simulations for coronary bifurcation stenting. The results obtained for John Doe already provide unique 3D insights that help to understand what is not shown by angiography and, for the moment, not yet with the same detail by intravascular imaging. For example, the impact of stent size selection and of performing POT are clearly illustrated within a realistic patient-based model, which may help to educate less experienced operators. Virtual bifurcation stenting also allows a comparison of multiple treatment options and strategies (stent sizes, stenting techniques, stent designs, etc.) for a specific patient, which is of course not possible in reality. Finally, insights into the impact of stenting and post-dilatation techniques on the local blood flow patterns can also be evaluated.
Another potential application is to combine the patient-specific computer modelling with virtual reality (VR) simulators such as the Coronary Pro (Mentice, Gothenburg, Sweden) and the ANGIO Mentor from Simbionix (3D Systems, Cleveland, OH, USA)16. These VR simulators comprise a library of complex patient pathologies and scenarios, which offers the opportunity for practising a variety of advanced interventional techniques using different devices. Recent advances in this field make it possible to import patient-specific anatomies for patient-based procedural training. Following a similar approach, it seems feasible to import the results of pre-computed patient-specific computer simulations, which would allow the study in detail of several realistic procedural alternatives. Currently, these VR simulators seem not to take into account the device or vessel wall mechanics, and generally rely on projection methods to visualise stent placement. Finite element analysis has the potential to enhance the realism further and to serve as a technology to improve further the fidelity of VR simulators.
Many variations have been introduced for classic techniques such as provisional stenting, crush or culotte stenting. Many of these techniques have been evaluated using bench testing, but investigating the actual clinical benefit for all these technical variations by randomised clinical trials is extremely challenging and expensive. Therefore, another potential future application of patient-specific virtual bifurcation stenting is to perform virtual clinical trials (e.g., comparison of two stenting techniques). A first crucial step in this direction is of course to demonstrate that certain simulation output parameters such as shear stress or stress within the arterial wall predict, for example, neointimal hyperplasia. A study could be designed for this purpose, with the required baseline imaging to be able to perform virtual bifurcation stenting, and with intravascular imaging at follow-up to quantify neointimal hyperplasia. Similar studies have been performed in the past for non-bifurcation stenting, for example, linking shear stress with in-stent restenosis17. However, several technical issues need to be addressed before such a validation study for virtual bifurcation stenting could be initiated. This type of computer modelling currently requires the collaboration of several engineering groups, and manual operations and lengthy computer simulations make large-scale studies challenging. Further attempts to automate and speed up this process are therefore required.
If the previously mentioned validation study were to prove the predictive power of virtual stenting, then there is also a future for patient-specific virtual bifurcation stenting to become a preoperative planning tool. With computer modelling, a physician could try several techniques and devices before treating the actual patient and, based on the results, determine the optimal treatment for that specific patient.
Conclusion
Virtual bifurcation stenting and flow modelling starting from patient-specific imaging is feasible and offers unique insights for treating coronary bifurcation lesions. For the moment, this technology can be used for educational and research purposes, and could be combined with virtual reality simulators. Clinical application such as virtual trials and preoperative planning require a thorough clinical validation of the predictive power of these computer simulations.
Funding
J. Wentzel and C. Chiastra are supported by the ERC starting grant (310457, BioCCora)
Conflict of interest statement
Y. Louvard has received honoraria from Medtronic and Terumo for workshops. P. Mortier and M. De Beule are shareholders of FEops, a spin-off from Ghent University providing consultancy services to the medical device industry. G. De Santis is an employee of FEops. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Fluid dynamics in terms of animated pathlines at peak flow rate for the provisional stenting and POT models. In the provisional stenting, particles are flowing faster and pathlines through the malapposed struts are clearly visible.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Fluid dynamics in terms of animated pathlines at peak flow rate for the provisional stenting and POT models. In the provisional stenting, particles are flowing faster and pathlines through the malapposed struts are clearly visible.