
“Medicine is a science of uncertainty and an art of probability” – Sir William Osler.
The overall numbers of percutaneous coronary interventions (PCI) of bifurcation lesions continue to increase worldwide. This is mainly due to an increased number of PCI procedures on the left main stem attributed to changing guidelines and increased life expectancy and thus an increase in the proportion of elderly, fragile patients with chronic heart diseases, turned down for surgery1. PCI of coronary bifurcations remains challenging and, despite refinements of stenting technique and the development of new generations of stent platforms that have a more favourable risk profile, a high rate of subsequent adverse clinical events is still present1. This increased risk of stent failure in bifurcation stenting is multifactorial, but stent deformation introduced by these techniques is one of the main causes of stent failure. There is a need to evaluate the impact of technical issues on the risk of late adverse events.
The coronary arteries divide in a fractal manner. The diameter of the branches correlates to the physical principle of minimal workload2. As a consequence of these underlying biological principles, the coronary vessels taper. This phenomenon is most prevalent after the take-off of a side branch, resulting in discrepancy in vessel diameter between the proximal vessel and the distal vessels in a bifurcation. Consequently, the original tubular manufactured stent needs to be changed to match the tapered anatomy of the coronary vessel. Thus, the stent may be deformed beyond the design specifications, and the integrity of metal, polymer and drug can be hampered. Bifurcation PCI can be performed very simply but can also be very complex and technically demanding1. The complexity of the stenting technique correlates to the number of technical issues during implantation (stent malapposition, crushed stents, heavy burden of metal and polymer in certain areas of the stented segment, as well as undersized stents and floating stent struts over the side branch ostium). Most of the technical issues related to stent failure impair flow through the device and into the vascular bed. Accordingly, flow disturbances may lead to adverse late outcomes, and thereby be a marker of increased risk of adverse clinical events1. Consequently, there is an unmet need for focus on technical issues in bifurcation stenting when aiming to improve the long-term efficacy and safety of the stents and procedures in the clinical setting.
In this issue of EuroIntervention, Toth and colleagues3 present a technical evaluation of a refinement of the culotte double stenting technique for bifurcation in comparison with conventional double stenting techniques.
They use a variety of modalities in the technical evaluation and include an elegant overall functional evaluation in terms of computational fluid dynamics (CFD). This computational simulation adds value to the evaluation of the device and improves the understanding of the influence of the technical modifications on the function (flow, blood and oxygen supply, workload) of the stented segment, and increases the understanding of the final interaction between the stented segment and the biological system.
Computational simulation has been known for years, but the recent exponential growth in computer power and the advances of supercomputers and their enormous computational simulation capacity make it highly probable that these methods and technologies will have an increasing role in evaluation of medical devices in the future.
Until now, computational simulation has been utilised with multiple methodologies to assess stresses with a range of computational power. This includes finite element analyses (FEA), CFD, wall shear stress (WSS), wall shear rate, vorticity and fluid structure interaction (FSI). These techniques answer distinct questions and have different limitations (that can also vary depending on computational power) and advantages, as outlined in Table 1.
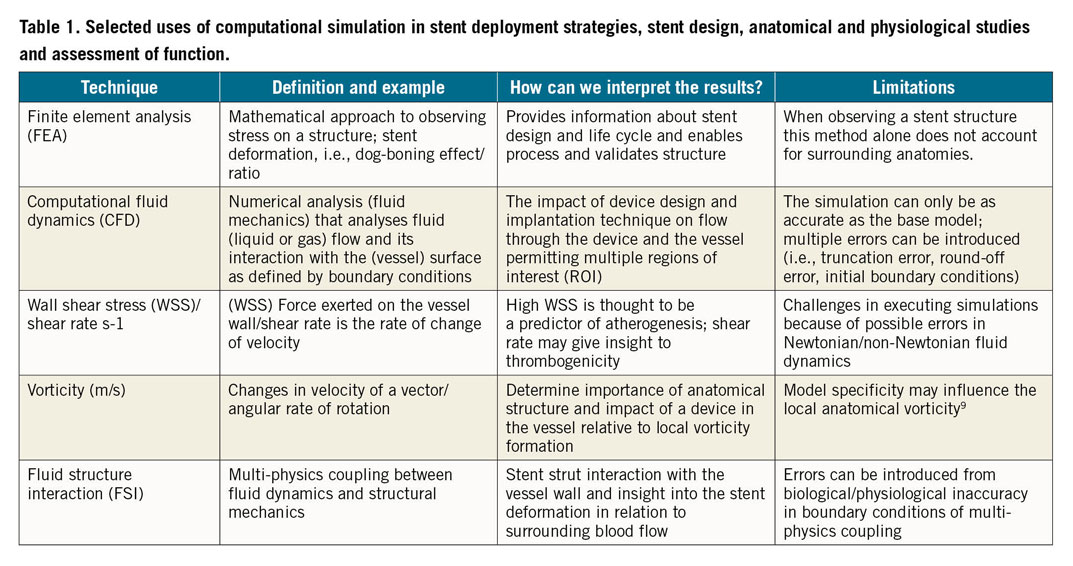
A way forward in this field is to refine what is already known and then, by recognising the limitations of the current techniques, develop the more complete data set required, paired with high performance computing (HPC), that will address questions more precisely in the future.
The past and the present of computational simulation
There exists a wide range of in silico testing modalities with a high level of utility for visualising and developing bifurcation stenting techniques. Multiple groups have developed bench testing in silicone tubes to test stent deployment physically4,5,6. These models have been a cornerstone for developing such complex techniques and standardising the consensus, as established by the European Bifurcation Club (EBC)1. Great efforts have been made to make these simulations translational to a clinical data set7. Bench testing and ex vivo imaging have been the basis of reconstruction for computational simulation utilising open source software and algorithms that are more complex and account for some variability in vessel compliance, cardiac output and disease states. This in turn has resulted in answering some of the questions relative to stent design and optimal stent deployment techniques and adding credibility to novel techniques, as elegantly described in the Toth paper. However, it is important to recognise the limitations of the current methodologies. The main concern is that the available models do not fully replicate the human anatomy, physiology and the human pathophysiology and, as a consequence, do not fully address the vessel wall-device (stent) interaction, and the effect of the device, on the patient over time.
The present and the future
INTEGRATING BIOLOGY, ANATOMY, PHYSIOLOGY, PATHOPHYSIOLOGY AND CLINICAL DATA IN THE SIMULATION MODELS
When interpreted correctly, simulations can tell us something that we may have predicted from intuition, for instance, that one may expect turbulent flow around a protruding stent strut; they may also provide validation for novel techniques. There is another opportunity for simulation to answer questions that may not be obvious and address the unknown unknowns in relation to long-term patient outcomes. A way forward is to build upon this foundation of simulation and to expand the computational power of multi-physics models that account for expansive elements in large parallel clusters. In turn, paired with the clinical data, these complex models could be utilised to understand better the behaviour of the bifurcation stenting in the anatomy and in the human pathophysiology and to see the whole picture as represented in a more detailed simulation. These models could also expand upon the boundary conditions of the vasculature and more accurately take into account soft plaques, calcification, pulsatile flow, vorticity and tortuosity, blood flow, comorbidities and time, thus representing the patient in four dimensions.
When moving forward in developing the current simulations and the future platforms, a standardised reporting of computational simulation in order to compare results among different studies needs to be addressed. One way forward could be consideration of a credibility strategy, as suggested in the methods of the Validation and Verification 40 consensus and advocated by the American Society of Mechanical Engineering (ASME). This approach would emphasise a template to observe associated risk and how a simulation is used to make informed decisions. This defines a purpose for the simulation, moving to assessing the risk of the model, prioritising the credibility threshold. As an example, these evaluations could be used to evaluate clinical uses that may not be described in the initial conditions of use but where perhaps the off-label use answers an unmet clinical need8.
In conclusion, computational simulation has the potential to be the tool of choice in evaluation of the different technical issues and their relation to function and outcome in bifurcation stenting. The advances of supercomputers can maximise the output and improve simulation by expansion. By including boundary conditions that are more precise, flow parameters and prediction of thrombosis as part of the models, the possibilities to test conditions of use for medical devices and simulate more realistic anatomy and physical conditions are wide open. By following this path, the way is clear for true integration of anatomy, physiology and device interactions in simulations which finally mimic the laws of nature.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

