Abstract
Virtual bench testing is a numerical methodology which has been applied to the study of coronary interventions. It exploits the amazing growth of computer performance for scientific calculation and makes it possible to simulate very different and complex multiphysics environments and processes, including coronary bifurcation stenting. The quality of prediction from any computer model is very sensitive to the quality of the input data and assumptions. This also holds true in stent virtual bench testing. This paper reviews the state of the art in the field of bifurcation stenting modelling and identifies the current advantages and limitations of this methodology.
Introduction
Virtual bench testing is a methodology currently used to test and characterise stent deployment by using a number of computer simulation techniques. As such, it parallels and integrates the traditional in vitro bench testing. Virtual bench testing allows the assessment of quantities that are impossible to measure experimentally, such as the stent and the arterial wall stress state and the wall shear stress. Recently, increasing efforts have been made to improve, optimise and automate computer simulations for the planning of bifurcation stenting1-7.
This paper discusses: i) the physics included in virtual bifurcation stenting (i.e., structural, fluid dynamics, and drug release), ii) the verification and validation of virtual stenting, and iii) the implications of virtual stenting in research and clinical practice.
The physics of virtual bifurcation stenting
The first step for a virtual bench test is the definition of the arterial vessel and stent geometry. The vessel can be either “idealised” or “population-specific” or “patient-specific”. In any case, the main dimensions of the model should be representative of a real case, with real diameters and lumen stenosis. Examples of “population-specific” studies are those by Williams et al8 and Girasis et al9, who used data from a population, such as the vessel diameters, the bifurcation angle, and the degree of stenosis, to define their models. The stent geometry can be reproduced easily due to significant improvements in computer-aided design (CAD) software, making any difference between the virtual reconstruction and the actual picture of the stent barely noticeable (Figure 1)10. Once the geometry is defined, structural, fluid dynamic or drug release virtual bench tests can be carried out.
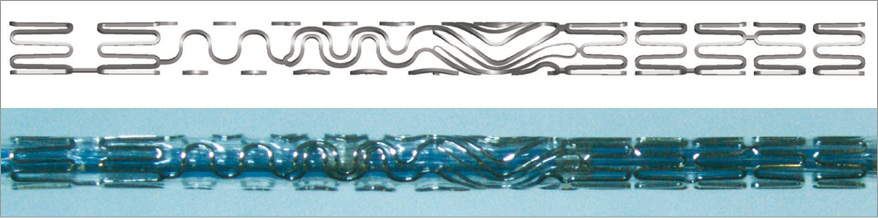
Figure 1. Tryton stent. Top: virtual model of a Tryton stent (Tryton Medical, Inc.) reconstructed with the CAD software SOLIDWORKS (Dassault Systèmes SOLIDWORKS Corp., Waltham, MA, USA). Bottom: a real Tryton stent crimped on the catheter.
Structural simulations require constitutive equations, i.e., the equations that describe the response of the material to a specific mechanical stimulus, which are ascribed to the stents, the balloons, the arterial wall, and the plaque. The material mechanical properties of the stent are known from standard mechanical tests, while those of the balloons are generally adjusted to obtain a pressure-diameter relationship similar to the data provided by the manufacturer10,11. Although some studies have reported in vitro tests on tissue specimens12,13, describing the in vivo mechanical behaviour of plaque is still a challenging topic. Studies which take into account the different composition of arterial tissues and plaque are lacking. The progress of intravascular ultrasound (IVUS) and optical coherence tomography (OCT) has substantially facilitated the simulation of plaque type and structure14,15. Recently, the inclusion of calcifications and the presence of lipid pool have been proposed by Conway et al16 on non-bifurcated simplified coronary arteries. The definition of the loading conditions, such as the inflation pressure of the balloon, completes the preparation of the model.
For fluid dynamic simulations the main issues are related to the identification of appropriate boundary conditions to apply at the inlets and outlets of the bifurcated model. Indeed, pointwise velocity (the so-called “spatial velocity profile”) or pressure measurements are scarcely available for the coronary arteries of a patient. Although the temporal flow tracings in the parent vessel can be measured17 and a number of studies have reported such time tracings, blood flow distribution in daughter arteries is often missing. Indeed, the overall coronary peripheral resistances drive the flow distribution and might play an important role which can be taken into account in a simplified fashion with lumped parameter models8.
To study the elution of drugs from coated stents, models need to take into account the transport phenomena and pharmacokinetics. The prediction of spatio-temporal drug distribution in the arterial wall entails the simulation of polymer degradation and hydrolysis kinetics, drug diffusion in the coating and the arterial wall, with reversible binding in the latter. The required input data are scarcely measurable in a coronary-like set-up, as they also depend on the properties of the surrounding arterial wall, which in turn depends on the compressive radial forces generated by the stent deployment and heart beating. Some models are present in the literature18-23, but only a few are related to coronary bifurcation21-23.
Geometrical predictions can easily find clear evidence from post-deployment images: stent malapposition24,25, plaque and carina shift26, role of calcified rings on stent deployment27, and the new anatomical configuration of the bifurcation. Furthermore, additional information such as the stress and strain fields in the arterial wall and in the plaque can be visualised and quantified. Stent fracture has also emerged as a problem for in-stent restenosis in coronary bifurcations28. In this regard, structural simulations are definitely a key tool in quantifying the stent stress and strain amplitudes which are the most important factors in the fatigue process. Fluid dynamic simulations allow the calculation and the analysis of the wall shear stress distribution and the presence of flow disturbances, such as recirculation or stagnation zones. In addition, drug distribution over time in the arterial wall is the typical output of drug-release simulations, which could explain the results found, for example, by Nakazawa and colleagues29, who showed that the carina is more uncovered than other parts. Furthermore, the paper by Zimarino et al30 indicated that thrombosis is expected to be higher when a double drug-eluting stent (DES) strategy is used compared to a single DES strategy. These findings can certainly be investigated by combining structural, fluid dynamics and drug-release computer simulations as done, for example, by Cutrì et al22. Figure 2 depicts an example of results from structural, fluid dynamics and drug-release analyses in a computer model of a coronary bifurcation. Moving image 1 and Moving image 2 show a stent expansion with arterial stresses and blood inside a stented coronary bifurcation, respectively.
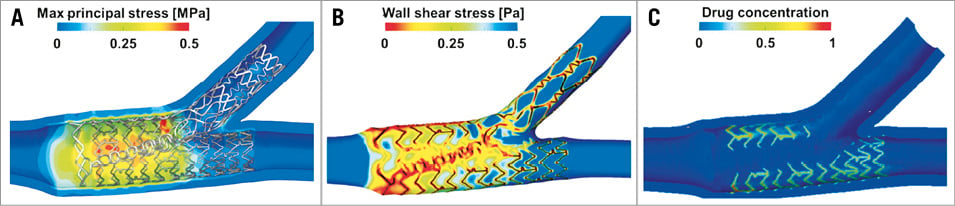
Figure 2. Examples of results from a virtual stenting of an idealised coronary bifurcation. A) Wall stresses (i.e., maximum principal stress in the arterial wall). B) Time-averaged wall shear stresses. C) Drug concentration patterns (images adapted from Morlacchi et al23).
The verification and validation of virtual bifurcation stenting
Verification is the process of building a model in the correct way, not only from the anatomical point of view, but also with regard to the accurate description of material properties, loads, constraints, and boundary conditions. From a geometrical point of view, the hypotheses and assumptions adopted should ensure that the model obtained closely resembles the real device, as shown in Figure 1. The boundary conditions need to be applied in order to represent the effects of the rest of the circulation or the loads generated by the balloon expansion correctly. The process of checking all the requirements must be implemented accurately before starting a simulation.
Validation is the process of determining whether a model is an accurate representation of the real system, with reference to the specific objectives of the investigation. Analysing the stent model of Figure 1, the expanded configuration obtained from the simulation has to be very close to the real expansion. The small dimensions of the coronary bifurcation diameters (2-4 mm) and the stent struts (80-100 µm) make in vivo fluid dynamic local measurements –such as velocities and shear stress– very difficult. Purposely designed in vitro bench tests can help in the validation process of virtual bench tests. As an example, images of a virtual and a real expansion of the Tryton stent (Tryton Medical, Inc., Durham, NC, USA) mounted on a stepped balloon are shown in Figure 3A. Mortier and colleagues11 validated their virtual bench tests in a similar way (Figure 3B). Raben et al31 used particle image velocimetry (PIV) flow measurements in in vitro stented coronary bifurcation models and compared them with the fluid dynamic results of the corresponding numerical models. The results were qualitatively in agreement, as both approaches successfully described the main features of the fluid flows for the different stenting procedures (Figure 4).
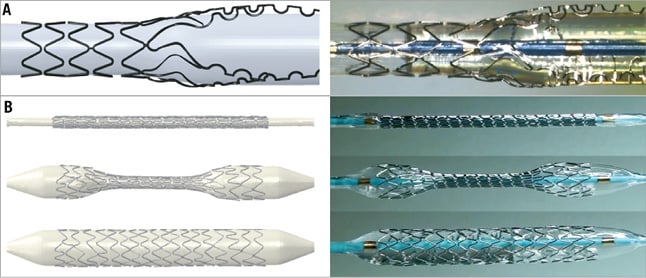
Figure 3. Stent free expansion. A) Virtual (left) and real (right) expansion of the Tryton stent (Tryton Medical, Inc.) (modified with permission from Chiastra et al10). B) Virtual (left) and real (right) deployment of the Integrity stent (Medtronic, Minneapolis, MN, USA) (reprinted with permission from Mortier et al11).
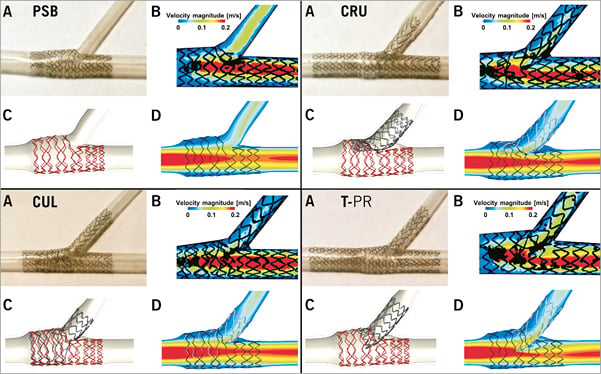
Figure 4. In vitro (A & B) and virtual (C & D) bench testing for four different stenting techniques. Provisional side branch (PSB) stenting, culotte technique (CUL), crush technique (CRU), and T-stenting technique with high protrusion (T-PR). The experimental digital particle image velocimetry (PIV) measurements are shown in B images, while the computational fluid dynamics (CFD) numerical results are reported in the D images (modified from Raben et al31, with permission).
The intended use of virtual bifurcation stenting
Every computer model is developed to meet a set of requirements dictated by the intended use. Stent manufacturers typically use virtual bench testing to explore a range of different stent designs and to select the one which best meets the design and regulatory specifications. For these applications, virtual bench methodology is mainly a tool to increase productivity and reduce the “time to market” of medical devices. Thus, an “idealised” model of the coronary bifurcation largely suffices. On the contrary, when a virtual bench study is carried out to help identify possible causes of restenosis, the use of “population-specific” models of the coronary bifurcation9 becomes mandatory, in order to include variations in epidemiologic data (e.g., patient’s age, sex and life habits, diagnosis of diabetes), in the anatomy (e.g., vessel diameters, bifurcation angle, degree of stenosis), and in the stent type/design. When virtual bench testing is used to compare different stent deployment procedures in a preoperative planning phase, a “patient-specific” model of that patient is clearly needed2,5,32. In the latter case, the software suite where the virtual bench test is embedded –possibly a desktop application– should offer a full range of interactive capabilities. They should include Digital Imaging and Communications in Medicine (DICOM) import of the patient’s images, interactive pre-processing to obtain the 3D geometry of the coronary bifurcation, anatomic data measurements, simulation of the stent deployment and post-processing.
As an example, we refer the reader to the paper in this supplement publication on the “John Doe” program33. The above capabilities should be made available: i) without requiring any major expertise of the operator in handling the technical details needed to run numerical simulation, and ii) enabling the interventional cardiologist to receive output information within a reasonable time for clinical decision making (optimally real time). These two are the major challenges which still prevent a full take-up of the virtual bench testing opportunities in the clinical environment.
Conclusions
This review sought to outline the new knowledge made available by virtual bench testing for coronary bifurcations together with their main issues, with a major focus on the applicability of the models to clinical practice. Many computer scientists and biomedical engineers have been working to tackle these issues. An example is provided by the recent European-funded RT3S project (Real Time Simulation for Safer vascular Stenting, GA FP7-2009-ICT-4-248801) to provide the clinician with a quantitative indication of the risk of stent fatigue fracture in peripheral arteries. The project developed a suite of virtual bench testing models –which included patient-specific and implant-specific factors– able to provide the stresses and strains induced in the stent by cyclic leg movements by means of pre-computed simulations. Similar approaches are also needed for coronary stenting.
Funding
C. Chiastra is supported by an ERC starting grant (310457, BIOCCORA). Y.S. Chatzizisis is supported by the Marie Curie International Reintegration Grant (249303, European Commission, Framework Program 7).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Stent expansion; simulation of the Tryton-based culotte technique.
Moving image 2. Fluid dynamics; animated pathlines in a coronary stented bifurcation at peak flow rate.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Stent expansion; simulation of the Tryton-based culotte technique.
Moving image 2. Fluid dynamics; animated pathlines in a coronary stented bifurcation at peak flow rate.