Abstract
The Visible Heart® Laboratory is an original experimental laboratory in which harvested animal hearts are resuscitated and connected to a support machine in order to beat outside the animal body. Resuscitated animal hearts may be exposed to various types of endovascular intervention under full, multimodality inspection. This unique experimental setting allows the performance of percutaneous coronary intervention (PCI) in a setting which resembles a standard catheterisation laboratory set-up, and contemporaneously allows unique multimodality imaging. For these reasons, the performance of PCI on bifurcations in the Visible Heart® Laboratory may improve the knowledge of the dynamic stent deformations and stent-vessel wall interactions associated with the different steps of the various techniques for bifurcation stenting. Furthermore, the collected images may also serve as a novel educative resource for physicians. The performance of bifurcation stenting in the Visible Heart® Laboratory is a promising experimental setting to gain novel information regarding any existing or future PCI technique to treat coronary bifurcations.
Introduction
The full comprehension of what may happen in coronary arteries during percutaneous coronary interventions (PCIs) continues to be an area of interest. Coronary bifurcated lesions, although being a frequent target for PCI, represent a complex subset of lesions. A growing number of techniques1 have been developed in order to treat bifurcated lesions with tubular stents using a variety of technical steps. Most of the improvements in the field of PCI of bifurcated lesions have been achieved by simulating, in bench tests, the response of tubular stents to the specific technical steps ideated for each technique2. More recently, due to improved digital computational potential, computer modelling has entered the field of bifurcation stenting simulation2. As a consequence, images generated by bench tests, micro-computed tomography and computer simulations represent a relevant source of knowledge for interventional cardiologists practising bifurcation PCI. However, the direct visualisation of coronary arteries during stenting procedures remains a dream for any operator who is practising complex endovascular techniques such as those required to stent coronary bifurcations. In the present paper, we report the outline of a novel project which has been developed in order to allow multimodality direct visualisation of the heart chambers and coronary arteries during bifurcation interventions using the Visible Heart® methodologies.
What is the Visible Heart® Laboratory?
The Visible Heart® Laboratory at the University of Minnesota is an experimental laboratory in which harvested animal hearts are resuscitated and connected to a support machine in order to beat outside the animal body. Resuscitated animal hearts may be exposed to various types of endovascular intervention under full, multimodality inspection.
The research protocol was specifically designed to ensure the humane treatment of all animals as indicated by the “Guide for the Care and Use of Laboratory Animals” and has been reviewed and approved by the University of Minnesota Institutional Animal Care and Use Committee.
Male swine (70-90 kg) are initially anaesthetised via an intramuscular injection of Telazol (7 mg/kg), and then intubated, at which time they are administered the volatile anaesthetic isofluorane. Once a surgical plane of anaesthesia is sustained, a medial sternotomy is performed and a standard cardioplegia solution is rapidly administered (at ~150 mmHg via the aortic cannula) to depolarise the heart. Then, the isolated heart is reanimated using previously published Visible Heart® methodologies3-7. Briefly, the great vessels of the heart are cannulated and attached to the Visible Heart® apparatus. Each heart is then warmed to body temperature and perfused with a Krebs-Henseleit buffer3, and a native sinus rhythm is elicited following defibrillator shocks (Figure 1, Moving image 1). Importantly, since the buffer utilised in these studies is clear, intracardiac imaging of the coronary vasculature is possible with optic devices. Endoscopic moving images of the aorta and heart chambers are obtained by employing 4 mm and 6 mm diameter endoscopes (IplexFX; Olympus Corporation, Tokyo, Japan). Intracoronary endovascular imaging is obtained using a 2.4 mm diameter fibrescope (Olympus, Center Valley, PA, USA) while standard fluoroscopic equipment (Ziehm Imaging, Nuremberg, Germany) is used to obtain angiographic images and guide the performance of angiography-based interventions as in human catheterisation laboratories.
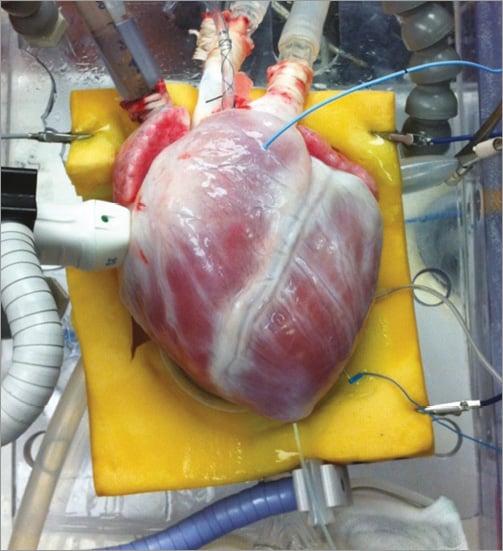
Figure 1. The harvested heart during reanimation in the Visible Heart® Laboratory.
Such Visible Heart® methodologies may be used to obtain endoscopic footage of coronary artery stent implantations within reanimated swine hearts. This experimental approach provides unique imaging of the device/tissue interface which can be used to increase knowledge in the field of bifurcation stenting procedures by allowing direct visualisation of stent deformation during each step of any bifurcation stenting technique. The images obtained could also be employed as useful training and educational tools since the Visible Heart® Laboratory at the University of Minnesota also maintains the free access website, the Atlas of Human Cardiac Anatomy (http://www.vhlab.umn.edu/atlas/).
Percutaneous coronary interventions on coronary bifurcations performed in the Visible Heart Laboratory
The Visible Heart® Laboratory has the unique feature of allowing the performance of PCI in a setting which resembles a standard catheterisation laboratory set-up, and contemporaneously allowing a unique multimodality imaging. In this laboratory, the reanimated heart is placed on a radiotransparent operating table, and the operator may rotate the radiologic C-arm equipment in order to achieve different angiographic views. Since the aortic arch is harvested along with the heart, a silicon tube terminally connected with the aortic arch mimics the descending aorta and allows for the insertion of guiding catheters, creating a setting for interventions which perfectly reproduces the femoral approach for PCI (Figure 2). Under fluoroscopic guidance, the operator may reach the ascending aorta with a 0.035” Teflon wire and place the guiding catheter (size and shape selected according to the specific heart/aorta size) into the left main or the right coronary artery ostium. Of note, in the pig’s heart selected for such interventions, extra back-up 3.5 to 4 and Judkins Right 4 curves usually fit perfectly, thus providing an interventional setting which perfectly mimics the daily clinical practice of PCI in human catheterisation laboratories. Once the coronary artery is cannulated by the guiding catheter, standard contrast media-based coronary angiography may be performed, and standard PCI equipment (coronary guidewires, balloons and stents) is used to perform PCI. Throughout the PCI procedure, the fibrescope may be kept inside the coronary artery to record unique intracoronary angioscopic images.
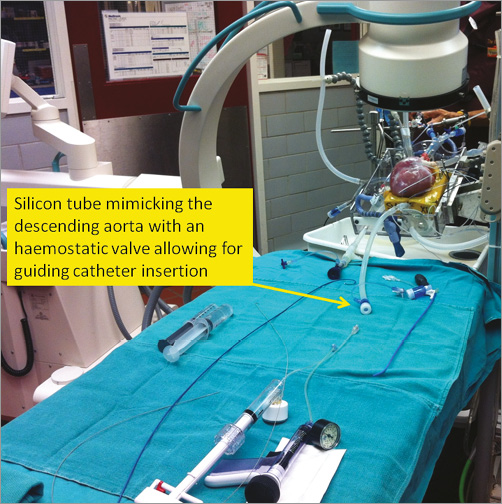
Figure 2. Typical set-up for percutaneous intervention in the Visible Heart® Laboratory.
The potential of the real-life conditions created during PCI performed in the Visible Heart® Laboratory is demonstrated by the documentation of edge dissection induced by too proximal balloon post-dilation as shown in Figure 3.
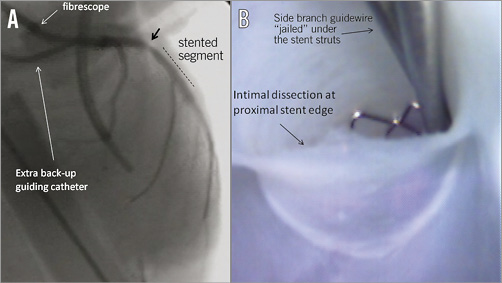
Figure 3. Edge dissection after stent post-dilation during percutaneous coronary intervention conducted in the Visible Heart® Laboratory. A) Angiographic image obtained during bifurcation intervention after incorrect, too proximal, balloon post-dilation of the main vessel stent. The black arrow shows a “hazy” lesion at the proximal stent edge. B) Angioscopic image obtained with documentation of intimal dissection at proximal stent edge caused by the incorrectly positioned post-dilating balloon.
Coronary bifurcations as seen in the Visible Heart Laboratory
As previously reported, the Visible Heart® Laboratory provides the opportunity to place various angioscopy probes for continuous imaging of the heart chambers and coronary arteries during PCI. Historically, coronary angioscopy is an imaging modality which has been available for more than 20 years and which has provided a pivotal contribution to the understanding of the natural history of coronary artery disease8. Coronary angioscopy allows direct visualisation of the luminal surface of the coronary artery, surface morphology of coronary lesions and qualitative details of a vessel’s surface which is not available by other means of intravascular imaging. Yet, coronary angioscopy in humans has important technical limitations, including the risk of causing damage to the arterial wall, disrupting coronary plaques, the inability to pass significant lesions, and the need for complete displacement of blood. Furthermore, due to its spatial occupation, coronary angioscopy in vivo cannot be applied to observe devices during implantation. Therefore, the Visible Heart® Laboratory model allows for the continuous and live three-dimensional visualisation of functional cardiac and coronary anatomy. It is possible to visualise the diameters and angulation of the daughter vessels as compared to the proximal main branch and compare the imaging with simultaneously performed traditional coronary angiography. As shown in Figure 4 and Figure 5, a qualitative evaluation and direct visualisation of the bifurcation anatomy, such as the diameter and angulation of the side branch and distal main branch, is readily available. Concepts such as the anatomical aspect of a bifurcation “carina” at the level of the side branch take-off cannot be understood better than by direct visualisation by angioscopy (Figure 5). The main limitation of such a model based on pigs’ hearts is the absence of atherosclerosis and the increased vessel elasticity. Nevertheless, thanks to the generosity of organ donors, occasionally it has even been possible to evaluate diseased human coronary bifurcations in the Visible Heart® Laboratory (Figure 6).
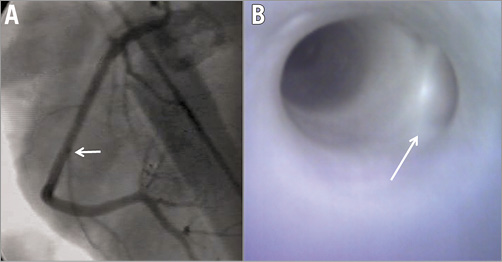
Figure 4. Bifurcation of right coronary artery with a small side branch. Coronary angiography (A) and angioscopy (B) demonstrating (white arrow) a coronary bifurcation with a small side branch and wide bifurcation angle.
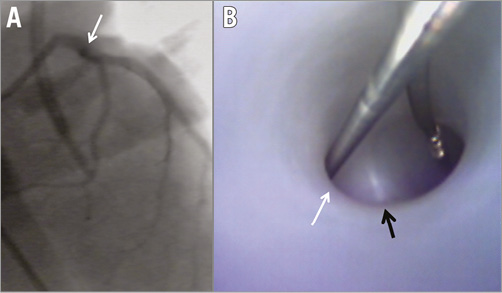
Figure 5. Coronary bifurcation with a large side branch and y-shape angle. Coronary angiography (A) and angioscopy (B) demonstrating a coronary bifurcation with a large side branch (white arrow) and y-shaped bifurcation angle (coronary guidewires placed in both branches of the bifurcation). The anatomic aspect of the bifurcation carina is immediately discernible by the angioscopic image (black arrow).
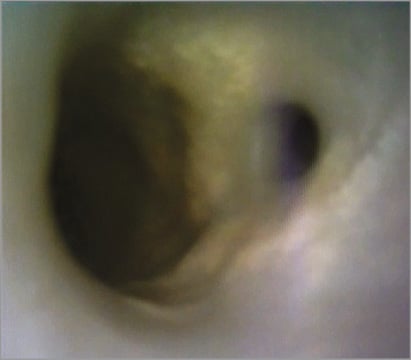
Figure 6. Angioscopy of coronary bifurcation of a reanimated human heart. Angioscopic image of a human coronary bifurcation with a small side branch demonstrating atherosclerotic plaque involving both branches of the bifurcation. The performance of bifurcation PCI on this model may add significantly to the understanding of the impact of plaque on device/technique/vessel interaction.
What we may learn from Visible Heart Laboratory in bifurcations
The greatest value of the Visible Heart® Laboratory as regards bifurcation is the ability to observe and document device/anatomy and technique/device/anatomy interactions in real time. Simulations of bifurcation stenting techniques in this model may be set at an impressive level of both reality and assessment since interventions are performed through standard guiding catheters on a beating heart and device/anatomy interaction may be visually checked (without any processing) continuously. Such experimental conditions by far outperform previous bench air models, which are flanked by the artificial material mimicking the vessel wall.
Potentially, one key benefit of the Visible Heart® Laboratory is the chance to practise and compare the different bifurcation techniques. Indeed, metal burden, vessel wall stent coverage, carina preservation, and stent deformity obtained with each technique may be directly visualised dynamically. By using this information, the benefits and the limitations of each step of each technique may theoretically be appreciated according to different anatomic conditions (different size and take-off angle of side branches, etc.). Furthermore, since the collected material may be shared using institutional websites, the Visible Heart® Laboratory may also serve as a novel educative resource for physicians interested in furthering their understanding of complex bifurcation techniques.
Acknowledgements
This work was funded in part by the Institute for Engineering in Medicine at the University of Minnesota and by Medtronic Inc.
Conflict of interest statement
F. Burzotta has been involved in advisory board meetings for Medtronic and has received speaker’s honoraria from Medtronic. B. Cook is employed by and has ownership interest in Medtronic Inc. P. Iaizzo and J. Singh have consulted for Medtronic Inc. Y. Louvard received honoraria (Visible Heart programme and proctorship) from Terumo. A. Latib serves on a Medtronic advisory board.
Online data supplement
Moving image 1. The reanimation of human heart 175, which was deemed not viable for transplant and was donated for research (via Lifesource). The heart was recovered using standard cardioplegia procedures, the great vessels were cannulated, and then the heart was placed on the Visible Heart® apparatus. Warmed clear perfusate was circulated through the heart, and subsequently the atria began to contract and the ventricles fibrillated. Upon the whole myocardium reaching a temperature of 37°C, the heart was defibrillated using external patch electrodes (34 J): a native sinus rhythm was initiated. (This Moving image may also be downloaded from the free access website, the “Atlas of Human Cardiac Anatomy”, http://www.vhlab.umn.edu/atlas/).
Supplementary data
To read the full content of this article, please download the PDF.
The reanimation of human heart 175, which was deemed not viable for transplant and was donated for research (via Lifesource). The heart was recovered using standard cardioplegia procedures, the great vessels were cannulated, and then the heart was placed on the Visible Heart apparatus. Warmed clear perfusate was circulated through the heart, and subsequently the atria began to contract and the ventricles fibrillated. Upon the whole myocardium reaching a temperature of 37°C, the heart was defibrillated using external patch electrodes (34 J): a native sinus rhythm was initiated. (This Moving image may also be downloaded from the free access website, the Atlas of Human Cardiac Anatomy, http://www.vhlab.umn.edu/atlas/).