Abstract
Aims: Smaller studies have previously shown promising results after Tryton Side Branch Stent™ (Tryton Medical, Durham, NC, USA) placement. However, these previous studies were limited by their small sample size and relatively short follow-up. We performed a patient-level pooled analysis to evaluate six-month and one-year clinical outcomes of more than 900 patients who were enrolled in eight registries with the Tryton stent.
Methods and results: Data from eight Tryton registries, including 905 patients with 929 bifurcation lesions, were pooled on a patient level to form one dataset. The primary outcome was six-month target vessel failure (TVF), defined as the composite of cardiac death, any myocardial infarction, and clinically indicated target vessel revascularisation. Procedural success was defined as successful stent placement and no in-hospital major adverse cardiac events. Multivariable analysis was performed to determine independent predictors for one-year TVF. Follow-up data were available in 97%. Procedural success was 95% and TVF rate was 6.5% at six months and 8.5% at one year. Stent thrombosis occurred in 0.5% of patients. Left main coronary artery bifurcation lesion (HR 6.46) and main branch reference vessel diameter <3.0 mm (HR 2.62) were independent predictors for TVF.
Conclusions: In the real world setting of registries including more than 900 patients, the use of the Tryton stent is associated with procedural and mid-term clinical results that compare very favourably with historical studies. The primary endpoint of TVF was primarily determined by reference vessel diameter of the main branch and left main bifurcation lesion location.
Introduction
Bifurcation lesions account for up to 20% of all coronary lesions treated by percutaneous coronary intervention (PCI)1,2. Based on multiple randomised trials3-5, the preferred treatment strategy is placement of a single stent in the main branch (MB), without side branch (SB) stenting unless indicated (“provisional single stent” approach)6. Nevertheless, PCI of bifurcation lesions is still associated with an increased risk of in-stent restenosis (ISR) and stent thrombosis (ST), even when drug-eluting stents (DES) are used7,8. Several dedicated bifurcation stents have been developed to improve clinical outcomes after PCI of bifurcation lesions. The Tryton Side Branch Stent™ facilitates a two-stent approach, employing the culotte technique. The stent consists of three zones: a distal zone, placed in the SB, provides optimal scaffolding of the SB ostium, a proximal MB zone, “anchoring” the stent in the MB, and a transition zone, connecting both zones, designed to provide complete carinal coverage while minimising the number of stent struts in the MB, with easy delivery of a regular stent in the MB (Figure 1)9.
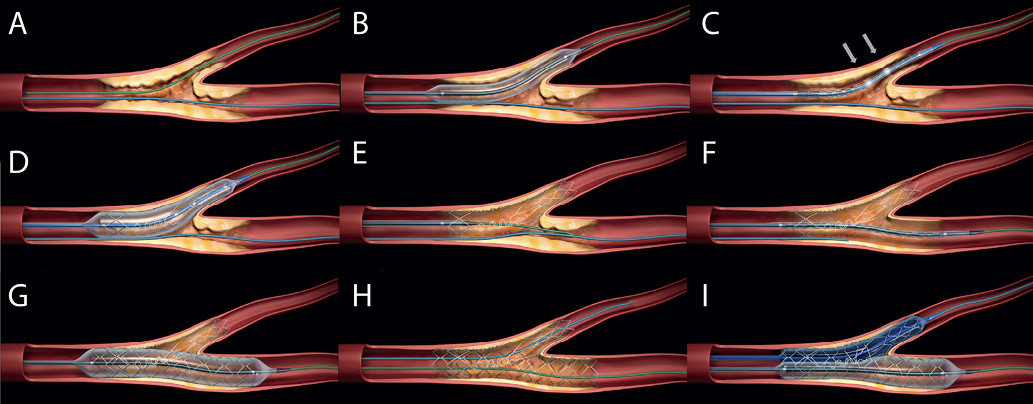
Figure 1. Deployment sequence of the Tryton Side Branch Stent for a de novo bifurcation lesion. (A) First, two guidewires are placed in both main and side branch. (B) Predilatation of the lesion is performed according to the operator’s discretion. (C) Positioning of the Tryton stent is done by placing the stent in such a way that the carina is in-between the two middle radiopaque markers (arrows). (D) The stent is deployed after which the delivery system is removed. (E) A deployed Tryton with its tri-ZONE™ design: a proximal zone “anchoring” the stent in the main branch, a transition zone with a specific geometry which facilitates easy main branch stent delivery, and a distal zone, covering the ostium of the side branch. After deployment, the guidewire in the side branch is retracted and advanced into the proximal main branch. (F, G and H) A workhorse stent is positioned and placed in the main branch. (I) The procedure is completed with final kissing balloon inflation.
Previous smaller studies with the Tryton stent have shown promising procedural and clinical outcomes10-13. However, these studies were limited by a small number of patients and a relatively short follow-up (six months). We performed a patient-level pooled analysis of all available registries to evaluate whether these results could be confirmed in a larger sample size, and to evaluate clinical outcomes beyond six months.
Methods
DEVICE
The Tryton Side Branch Stent™ has been described previously9. In short, it is a 5 Fr- or 6 Fr-compatible balloon-expandable, cobalt-chromium bare metal stent. Its design uses the Tri-ZONE™ technology: a distal zone scaffolding the SB, a transition zone at the carina with three panels designed to accommodate the wide range of carinal anatomy, and a MB zone with a minimal amount of metal allowing easy delivery of a standard workhorse stent. Before placement, guidewires are placed in both the MB and the SB. The Tryton stent is then placed using four radiopaque markers on the stent delivery system for optimal positioning. After stent deployment, the SB guidewire is withdrawn to the Tryton transition zone and advanced into the MB. A standard DES is placed in the MB, “jailing” the SB. The procedure is completed with refenestrating a guidewire into the SB to perform final kissing balloon inflation (Figure 1).
IDENTIFICATION OF REGISTRIES
The collaborative analysis was initiated by the Academic Medical Center - University of Amsterdam, The Netherlands. Tryton Medical was contacted and asked which registry studies, to their knowledge, were performed or are still ongoing utilising the Tryton stent. Furthermore, a literature search was performed using MEDLINE and EMBASE, from August 2006 to January 2012, by using terms including: “side branch stent”, “Tryton”, and “facilitated culotte”. In addition, manual searches were performed using reference lists from review articles and published abstracts from Scientific Sessions of the American Heart Association, American College of Cardiology, European Society of Cardiology, Transcatheter Cardiovascular Therapeutics, and EuroPCR, from August 2006 to December 2011.
Ten registry studies were identified which included consecutive patients who had been treated with the Tryton stent. In five registries, enrolment was closed and follow-up completed. Three other (single-centre) studies were identified which were still enrolling. From these ongoing registries, all patients who had been treated with the Tryton stent before January 2012 were included in the current analysis. From the remaining two registries, enrolment and follow-up status were unknown. An overview of all eligible registries compiled using the Tryton stent is outlined in Table 1.
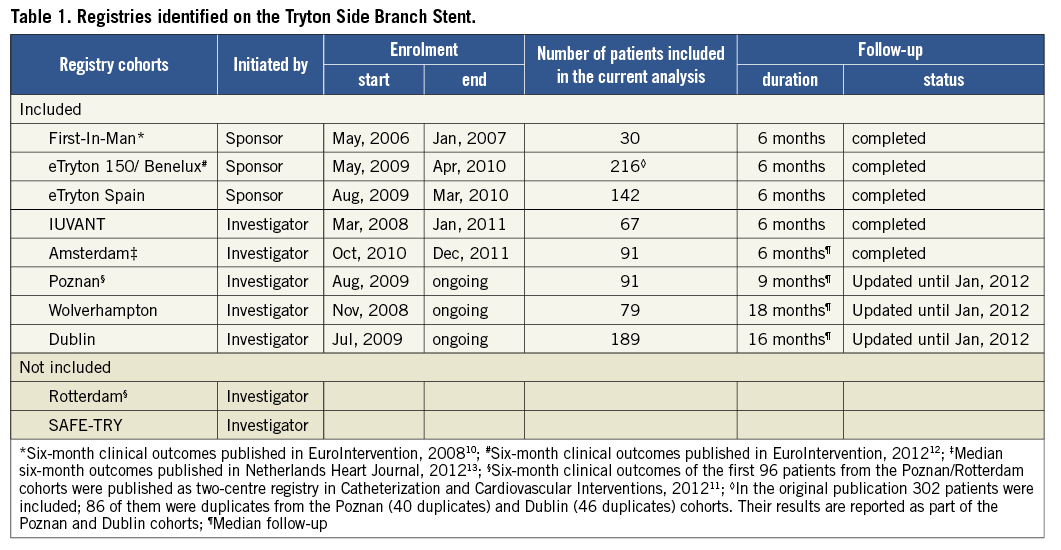
DATA REQUESTED
Investigators involved in the ten registries were contacted and requested to provide individual patient data to form a pooled patient database. An analytical plan was developed including core variables on demographics, clinical history, risk factors for coronary artery disease, and lesion and procedural characteristics. The investigators were requested to provide individual clinical outcomes. If registries were still ongoing, the investigators were requested to extend patient follow-up until January 1st, 2012. Investigators of eight registries agreed to participate in the current analysis. Datasets from these eight registries were sent for merging to the co-ordinating centre at the Academic Medical Center in Amsterdam. Each investigator vouched for the correctness of the data.
MERGING OF DATASETS
After receiving individual patient data, an assessment was performed to identify duplicates in the different datasets, using date of birth, date of procedure and gender. We identified 86 duplicates, of which 40 were found in the Poznan and eTryton 150/ Benelux registries, and 46 in the Dublin and eTryton 150/ Benelux registries. From these duplicates, patient data from the Poznan and Dublin cohorts were used, since longer follow-up was available in these cohorts compared with the six-month follow-up of the eTryton 150/ Benelux registry. Hereafter, datasets were merged to form one pooled patient database.
CLINICAL OUTCOMES
The primary outcome was target vessel failure (TVF), defined as the composite of cardiac death, any myocardial infarction (MI), and clinically indicated target vessel revascularisation (TVR) at six months. Secondary outcomes were TVF at one year, procedural success (defined as successful stent deployment at the site of the target lesion and no major in-hospital complication such as death or MI), and any death, cardiac death, any MI (including both periprocedural and spontaneous MI), any revascularisation, any target lesion revascularisation (TLR), clinically indicated TLR, any TVR, clinically indicated TVR, ST (subdivided into definite and probable), and the composite of death and MI at six months and one year. Clinical outcome adjudication was in accordance with the definitions of the Academic Research Consortium14.
STATISTICAL ANALYSIS
Continuous variables are reported as mean (± standard deviation) or median [interquartile range], where appropriate. Categorical variables are presented as frequencies (%). Cumulative event rates were estimated using the Kaplan-Meier method. Follow-up was censored at the last known date of follow-up or at one year, whichever came first. To determine independent predictors of TVF at one year, we performed univariable Cox regression analyses using all available baseline clinical, angiographic and procedural variables. If a variable had more than 5% cases with missing values, an indicator of “missingness” was created and included in the Cox regression models. Variables significantly associated with TVF by univariable analysis (p<0.1) were subsequently entered in a multivariable Cox regression model. Subsequently, these analyses were repeated to determine predictors of TLR and the composite of death and MI. A p-value <0.05 in the multivariable analysis was considered statistically significant. All analyses were performed using SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA). The continuous variables age, lesion length and reference vessel diameter were entered into the models as dichotomous variables as follows: age >60 years, MB lesion length >20 mm, SB lesion length >10 mm, MB reference vessel diameter (RVD) <3.0 mm, and RVD of the SB <3.0 mm. Categorical variables were made dichotomous as follows: thrombolysis in myocardial infarction (TIMI) flow as TIMI 0/1 versus TIMI 2/3, indication for PCI as acute coronary syndromes (ACS) versus no ACS, and bifurcation angle as bifurcation angle >80° versus ≤80°.
Results
BASELINE CHARACTERISTICS
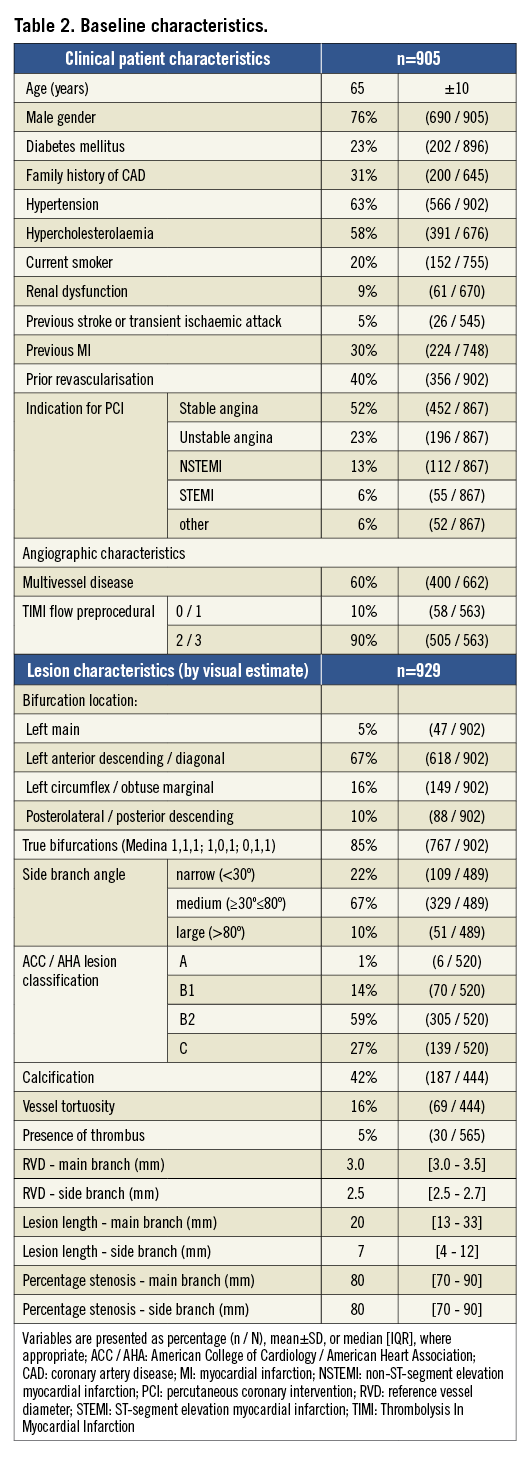
This study analysed 905 patients with 929 bifurcation lesions who were treated with the Tryton stent between May 2006 and January 2012. Patient baseline and angiographic characteristics are outlined in Table 2. The average age was 65 years, 76% were male, and 23% diabetic. Almost half (42%) of the patients included had ACS as an indication for PCI, of whom 23% had unstable angina, 13% non-ST-segment elevation myocardial infarction (NSTEMI), and 6% ST-segment elevation myocardial infarction (STEMI). Two patients had three Tryton-treated bifurcation lesions, while another 20 patients had two bifurcation lesions treated with the Tryton stent. Six hundred and eighteen (67%) bifurcation lesions were located in the left anterior descending (LAD) artery, and 47 (5%) in the left main (LM) coronary artery. Eighty-five percent of the lesions were true bifurcations with involvement of both MB and SB (i.e., Medina 0,1,1 or 1,0,1, or 1,1,1), and in 109 (22%) cases the bifurcation lesions had a narrow angle (<30%). Median RVD of the MB and SB were 3.0 mm and 2.5 mm, respectively. Median length of the lesion in the MB was 20 mm, whereas that of the SB lesion was 7 mm.
PROCEDURAL CHARACTERISTICS
Procedural characteristics are outlined in Table 3. Predilatation of the SB was performed in 89% of the cases. The Tryton stent was successfully placed at the intended site in 907 (98%) bifurcation lesions. In 22 bifurcations, it could not be delivered at the intended site, mostly due to excessive calcifications. A bare metal stent was used as the MB stent in 21 (2.4%) bifurcation lesions and a DES in 853 (96%) lesions. The median length of the MB stent was 23 mm, with a median diameter of 3.0 mm. In 205 (32%) patients an additional stent was placed in the MB, whereas in 127 (14%) patients an additional stent was placed in the SB. In 26 (3.8%) lesions, additional stenting was required due to a dissection in the MB or SB. Final kissing balloon inflation was performed in 83% of the cases.
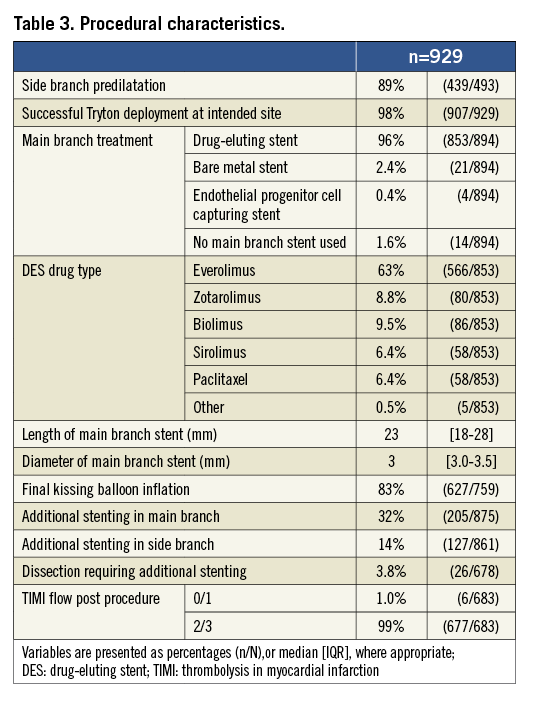
Procedural success was obtained in 862 patients (95%). In 43 patients procedural success could not be obtained because of unsuccessful Tryton delivery at the intended site in 22 cases, and in-hospital death or MI in 21 cases.
CLINICAL OUTCOMES
Six-month and one-year clinical outcomes are summarised in Table 4. Follow-up data were available in 875 (97%) patients. Median follow-up was 198 days [177-378]. Clinical follow-up of at least six months (±two weeks) was obtained in 707 (81%) patients. Cardiac death occurred in 14 patients. The six-month and one-year cumulative cardiac death rates were 1.6% and 1.8%, respectively. At six months, 26 patients suffered from an MI, whereas MI occurred in five additional patients between six months and one year, resulting in cumulative MI rates of 3.0% and 4.3%, respectively. Sixty (40 at six months) revascularisations occurred. Clinically indicated TVR was performed in 27 (3.3%) patients at six months and in 31 (4.4%) at one year. Target lesion revascularisation was performed in 34 patients, 28 of whom had a clinical indication, resulting in clinically indicated TLR rates of 2.9% and 4.0% at six months and one year, respectively. Four (0.5%) ST occurred, of which two (0.2%) were definite and two (0.2%) were probable.
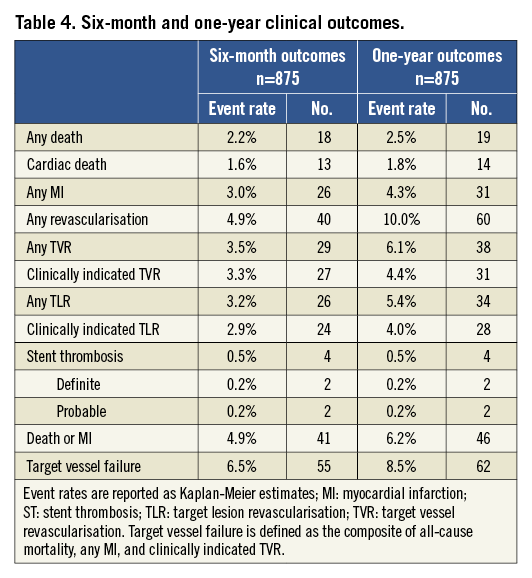
Overall, the cumulative rate of the primary composite outcome of TVF was 6.5% at six months and 8.5% at one year (Figure 2 and Figure 3).
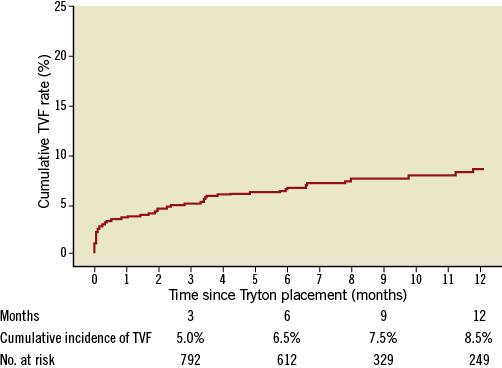
Figure 2. Twelve-month TVF rate. TVF: target vessel failure
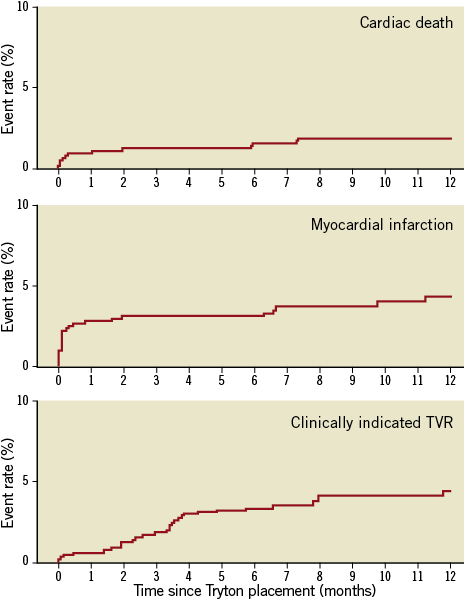
Figure 3. Twelve-month cumulative event rates of the individual components of target vessel failure; TVR: target vessel revascularisation
PREDICTORS OF TVF, DEATH/MI AND TLR
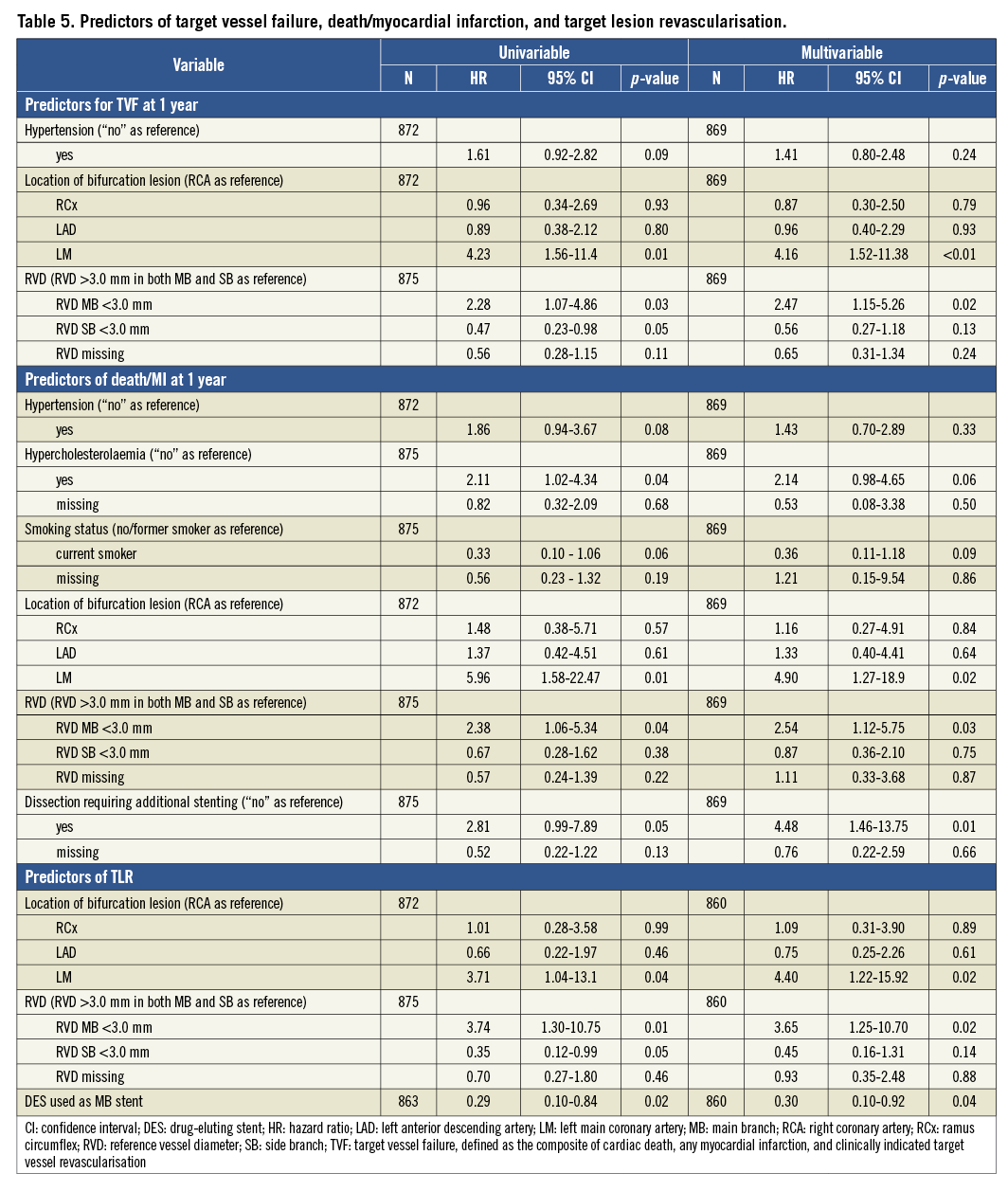
Univariable and multivariable Cox regression analyses are outlined in Table 5. In the univariable analyses, the following factors were identified as predictors of one-year TVF: hypertension, bifurcation lesion located in the left main coronary artery, RVD of the MB <3.0 mm, and RVD of the SB <3.0 mm. Multivariable analysis identified bifurcation lesions located in the left main (HR 4.16; 95% CI: 1.52-11.38; p<0.01) and RVD of the MB <3.0 mm (HR 2.47; 95% CI: 1.15-5.26; p=0.02) as independent predictors of one-year TVF. Bifurcation lesions located in the left main coronary artery, hypertension, hypercholesterolaemia, RVD of the MB <3.0 mm, dissection requiring additional stenting and current smoker were identified as predictors of death/MI. Left main location (HR 4.90, 95% CI: 1.27-18.9, p=0.02), RVD of the MB <3.0 mm (HR 2.54, 95% CI: 1.12-5.75, p=0.03), and dissection requiring additional stenting (HR 4.48, 95% CI: 1.46-13.75, p=0.01) were identified as independent predictors of death/MI at one year by multivariable Cox regression. Bifurcation lesion located in the left main, RVD of the MB <3.0 mm, RVD of the SB <3.0 mm and DES use for MB stenting were identified as predictors of TLR. Multivariable analysis identified bifurcation lesions located in the left main (HR 4.40, 95% CI: 1.22-15.92, p=0.02), RVD of the MB <3.0 mm (HR 3.65, 95% CI: 1.25-0.70, p=0.02), and DES use for MB stenting (HR 0.30, 95% CI: 0.10-0.92, p=0.04) as independent predictors of TLR.
Discussion
Our study provides procedural and clinical outcomes from eight registries encompassing real world data on Tryton stent implantation in more than 900 patients. Procedural success was obtained in 95%. Target vessel failure and clinically indicated TLR rates were 6.5% and 2.9% at six months and 8.5% and 4.0% at one year, respectively. The probable/definite ST rate was 0.5% at one year. The occurrence of the TVF composite outcome was primarily determined by a small (<3.0 mm) RVD of the MB and a left main coronary artery location of the bifurcation lesion.
COMPARISON WITH PREVIOUS CORONARY BIFURCATION STUDIES
A previous large Italian registry, which included 4,314 patients with coronary bifurcation lesions treated with DES or bare metal stents, reported a major adverse cardiac event (MACE) rate, defined as the composite of cardiac death, any MI, CABG, and TLR, of 13.1% at one year, with TLR and definite/probable ST rates of 10.2% and 1.4%, respectively15. Another large US DES registry, which enrolled 1,650 patients with coronary bifurcation lesions, showed a nine-month MACE (composite of any death, any MI, and TVR) rate of 10.9%, with TVR and definite/probable ST rates of 6.9% and 1.9%, respectively16. To date, six randomised studies have been performed comparing single-stent with two-stent techniques for coronary bifurcation lesion treatment. A meta-analysis of these trials showed a TLR rate of 5.3% and a ST rate of 0.8% in the single-stent group at six-month to twelve-month follow-up17. Finally, a bifurcation lesion substudy from a large randomised “all-comers” trial comparing a biolimus-eluting with a sirolimus-eluting stent showed one-year MACE (cardiac death, any MI, and clinically indicated TVR) rates of 12.8% and 16.8%, respectively. At one-year follow-up, clinically indicated TLR rates were 3.5% and 9.6%, while definite/probable ST rates were 3.5% and 3.3%18. Of note, the provisional single-stent approach was the preferred strategy in almost all patients enrolled in this all-comers trial. In summary, historical data demonstrated MACE/TVF rates (including cardiac and non-cardiac death, any MI, and TVR/TLR) of 11%-17%, (clinically indicated) TLR rates of 3.5%-10%, and probable/definite ST rates of 0.8%-3.5% at six to 12 months. Indeed, the clinical outcomes observed in our patient-level pooled analysis of eight registries on the Tryton stent (TVF 8.5%, clinically indicated TLR 4.0%, and a probable/definite ST 0.5% at one year) compare very favourably with those reported in the literature.
STENT THROMBOSIS
Historically, bifurcation lesions have been associated with a higher risk for ST5. Higher rates of ST in bifurcation lesions have several aetiologies. Incomplete stent apposition (ISA) occurred in more than 60% of bifurcation lesions treated when the two-stent crush technique was used19. Both ISA and non-apposed SB struts, defined as struts located at the ostium of a side branch without a vessel wall behind, are associated with delayed neointimal healing and incomplete endothelialisation20. Another potential cause of ST in bifurcations is excessive local drug delivery when two DES are used. In our analysis, we observed a very low ST rate. This could in part be explained by the tapered Tryton stent design, which obeys Murray’s law21, facilitating better strut apposition in the proximal MB. Second, due to the transition zone design, the number of stent struts is minimised at the carina level. Third, the same transition zone provides large cells for easy delivery of a regular stent in the MB whereas, in the classical culotte technique using two conventional stents, stent expansion and apposition are limited by the cell size through which the stent is placed22. Lastly, as a bare metal stent, Tryton avoids excessive drug delivery due to a double layer of DES struts, a factor that may reduce ST risk further.
PREDICTORS OF TVF, DEATH/MI AND TLR
In our analysis, bifurcation lesions located in the LM coronary artery were independently predictive of TLR, a composite of death/MI, and TVF. This could be due to several factors. According to the current European and American guidelines, CABG is the preferred treatment for unprotected LM coronary artery (ULMCA) disease, whereas PCI could be considered in high surgical risk patients16,17. Therefore, a subgroup of relatively high-risk patients, unsuitable for CABG surgery, could have been treated with the Tryton stent, resulting in higher death/MI rates at follow-up. Furthermore, higher TLR rates may also be explained by the angiographic follow-up, which is done routinely in most patients with PCI-treated ULMCA disease and is associated with a well-known increase in revascularisation rates23.
A second independent predictor for TLR, the composite of death/MI, and TVF in our study was a small (<3.0 mm) RVD of the MB. This finding is in agreement with previous studies investigating predictors for ISR7,24. A small RVD of the SB, on the other hand, was associated with a trend towards lower TLR and TVF rates. This seems counter-intuitive, but may be explained by several factors. As mentioned above, ULMCA was a strong predictor for TVF and TLR. The side branch of the ULMCA is by definition the circumflex artery, which has a RVD >3.0 mm in most cases. Indeed, if the analysis was repeated excluding the patients treated for ULMCA disease, the hazard ratio of SB RVD <3.0 mm for the prediction of TVF and TLR was closer to equivalence. Furthermore, repeat angiography was not performed in the majority of the patients, except in the FIM study and, partially, in the IUVANT study. It is conceivable that ISR of small side branches may occur without symptoms and does not result in revascularisation. This could have been the case in our patients. Indeed, no association was observed with death and MI. Third, even if ISR was observed, interventional cardiologists may have been conservative in the treatment approach of small restenotic SB. Lastly, the majority of patients were treated with the Tryton stent mounted on a stent balloon delivery system with SB diameter of 2.5 mm, which may have resulted in some malapposition in larger side branches. Recently, newer Tryton stent designs have been introduced, including SB diameters of 3.0 mm and 3.5 mm. Further studies are needed to evaluate whether this will improve clinical outcomes after treatment with Tryton in lesions with large SB diameters (including LM bifurcations).
STUDY LIMITATIONS
The study suffers from the usual limitations of registry data. The first limitation is the selection bias due to the lack of strict inclusion and exclusion criteria and to the fact that Tryton stent use was at the discretion of the operator in most of the analysed registries. Second, the majority of the data was not independently monitored which may have introduced inaccuracies in the data. Third, the lack of routine angiographic follow-up, except for the FIM study and partially for the IUVANT study, may have resulted in under-detection of angiographic ISR. However, angiographic follow-up is not routinely performed in clinical practice and results in an increase in clinically unjustified revascularisations23. Fourth, cardiac biomarkers were measured after PCI only if clinically indicated (i.e., ischaemic chest pain and/or new ischaemic changes on the electrocardiogram), resulting in potential under-reporting of periprocedural MI. However, the prognostic significance of periprocedural MI is a topic of ongoing debate25,26.
Conclusions
In a real world setting, including ACS patients presenting with MI in a considerable proportion of cases, use of the Tryton Side Branch Stent™ for treatment of coronary bifurcation lesions is associated with a 95% procedural success rate and clinical outcomes that compare very favourably with historical data.
Conflict of interest statement
M.J. Grundeken, M. Norell and J.J. Wykrzykowska receive consultancy fees from Tryton Medical. P.R. Stella, A. Bartorelli and M. Lesiak are members of the steering committee of the Tryton IDE trial. The other authors have no conflicts of interest to declare.

