Device description
Name and manufacturer: The Tryton Side Branch Stent; Tryton Medical, Durham, NC, USA.
Approval status: CE mark in 2008 (including left main indication in 2014), not approved outside Europe, Russia and the Middle East.
Platform: Cobalt-chromium.
Strut thickness: 84 microns (0.0033”).
Coating: Not coated, available in bare metal only.
Specific stent design: The stent consists of three zones: 1) the distal side branch zone with conventional stent design; 2) the transition zone with three independently deformable panels providing ostial scaffolding and coverage; and 3) the proximal main branch zone with two “wedding bands” facilitating mounting of the stent on the delivery balloon and with three undulating fronds minimising the amount of metal (Figure 1A).
Guiding: 5 Fr or 6 Fr (depending on stent size).
Classification according to MADS: Inverted culotte in the “A (inverted)” category.
Delivery system: Single rapid exchange delivery system with four radiopaque markers to guide positioning. The stent is balloon-expandable and mounted either on a straight or on a stepped delivery balloon (Figure 1B).
Stent sizes: The straight version has a diameter of 2.5 mm. The stepped version has diameters ranging from 3.0-4.0 mm (proximal) and 2.5-3.5 mm (distal).
Stent length: The original Tryton is 19 mm (18 mm for larger-sized Tryton stents) and the more recent “Tryton short” is 15 mm (shortening the proximal main branch zone) (Figure 1C).
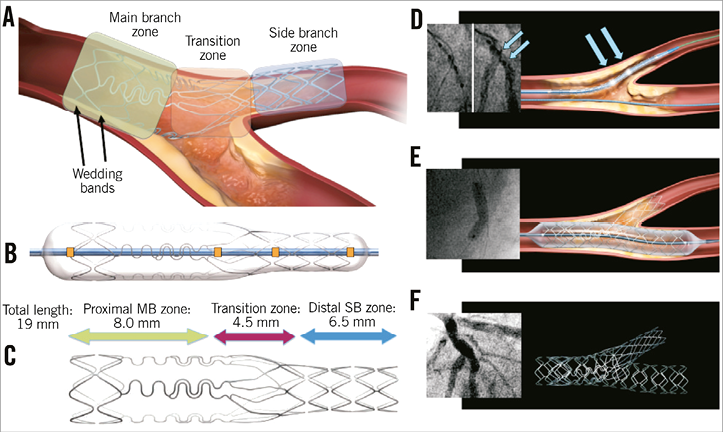
Figure 1. Tryton design and crucial procedural steps. Explanation of the panels can be found in the text.
Procedural details
After wiring both branches, predilations of main and side branch are performed according to the operator’s preferences. Tryton positioning is performed using four radiopaque markers on the delivery system: two markers indicating the distal and proximal stent edges and two middle markers indicating the transition zone (orange squares, Figure 1B). The stent should be positioned in such a way that the carina is positioned in-between the two middle markers (blue arrows, Figure 1D). After stent deployment, post-dilation of the proximal main branch zone using the proximal optimisation technique (POT) is recommended to ensure adequate apposition of the wedding bands and the panels of the transition zone. Then, the side branch guidewire is retracted and advanced into the distal main branch. Dilatation of the main branch is performed, according to operator preference. After retracting the “trapped” main branch wire, a regular drug-eluting stent (DES), sized according to the distal main branch diameter as recommended by the European Bifurcation Club, is advanced and deployed in the main branch (Figure 1E). Finally, it is recommended to perform another POT before finalising the procedure with final kissing balloon dilatation using non-compliant balloons (Figure 1F, Moving image 1, Moving image 2).
Clinical data
Multiple European registries, including the first-in-man study, consistently showed procedural success rates of 94-97.2% and encouraging clinical results1-6. Combined data from eight (published and unpublished) registries (n=925), pooled on a patient level, showed favourable outcomes at 1 year7. Target vessel failure (TVF) rate (composite of cardiac death, myocardial infarction [MI] and clinically indicated target vessel revascularisation [TVR]) was 6.5% at six months and 8.5% at nine months. One-year TVR rate was only 6.1%, and one-year stent thrombosis (ST) rate was low (0.5%)7.
Non-randomised intravascular imaging studies have shown less favourable healing patterns of the (bare metal Tryton-treated) side branch compared with the (DES-treated) main branch8-11. Although a single study suggested less favourable healing of the proximal main branch stent edge12, other studies have shown comparable healing between proximal and distal main branch, confirming that the Tryton with DES overlap does not negatively influence the healing of the proximal main branch8,10,11.
The Tryton stent was investigated further in the pivotal Tryton Investigational Device Exemption (IDE) randomised trial13. This was the first, and thus far only, randomised trial powered on clinical endpoints comparing a dedicated bifurcation stent with the provisional single-stent strategy. The primary non-inferiority endpoint of nine-month TVF, defined as the composite of cardiac death, target vessel MI and clinically driven TVR, was not met (17.4% in Tryton versus 12.8% in the provisional group, pnon-inferiority=0.42), mainly due to a numerically higher rate of periprocedural MI (3x ULN of CK-MB) in the Tryton arm (13.6% versus 10.1%, p=0.19)13. The main secondary endpoint, powered for superiority, was percentage diameter stenosis (%DS) of the side branch, which was slightly lower in the Tryton group (31.6% vs. 38.6%, p=0.002) when QCA was performed using the pre-specified per-protocol single-vessel QCA analysis. However, a post hoc QCA analysis using a bifurcation QCA algorithm did not show any difference between the two groups (34.8% vs. 32.0%, p=0.18)14. However, only ~40% met the inclusion criterion of side branch diameter >2.5 mm on visual estimate (~2.25 mm on QCA). It is conceivable that only larger side branches will benefit from a two-stent approach and a post hoc analysis indeed suggested a beneficial effect of the Tryton stent over balloon angioplasty in patients with side branch diameters >2.5 mm. (TVF in Tryton 11.3% vs. provisional 15.6%, p=0.383; Généreux, Washington, CRT 2014).
Data on the use of Tryton on left main bifurcation lesions are discussed elsewhere in this supplement15.
Ongoing studies
Currently a single-arm registry is ongoing, representing an extension of the IDE trial, including 133 patients. The main inclusion criterion is side branch diameter >2.25 mm confirmed by QCA. The primary endpoint is the rate of periprocedural MI (3x ULN of CK-MB) which will be compared with a pre-specified performance goal of 17.9% (based on the periprocedural MI rate in the provisional group [11.9%] plus a non-inferiority margin of 6%).
The NORDIC V study is currently running to investigate the role of final kissing balloon dilatation (FKBD) in the Tryton strategy16. In this study, 150 patients will be randomised 1:1 between routine FKBD and a strategy in which FKBD is only performed when side branch %DS is >50% or TIMI flow <3. Patients will return for an angiographic follow-up with OCT at eight months.
An effort is currently being made to collect long-term clinical follow-up (five to seven years) of patients included in the previously published patient-level pooled analysis7.
Unique features
– Only bifurcation stent specifically designed to facilitate an inverted culotte two-stent technique, offering potential advantages in scenarios where the side branch needs to be stented first.
– Special design with Tri-ZONE® Technology accommodating to a wide range of bifurcation anatomies and minimising the amount of metal in the main branch.
– Only device with a left main indication on its CE mark15.
Potential improvements
Drug coating: currently only available as bare metal stent.
Conflict of interest statement
P. Généreux received speaker fees from Abbott Vascular and consultancy fees from Cardiovascular Systems Inc. J. Wykrzykowska received speaker and consultancy fees from Abbott, Tryton Medical, Inc., and Stentys. M. Leon is on the scientific advisory boards of Abbott Vascular, Boston Scientific, and Medtronic; and is the principal investigator of the Tryton IDE trial. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Animation of the deployment sequence.
Moving image 2. Example of a Tryton case.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Animation of the deployment sequence.
Moving image 2. Example of a Tryton case.