Abstract
Aims: To report serial intravascular ultrasound (IVUS) findings of bifurcation lesions treated with the dedicated Tryton Side Branch Stent to assess mechanisms of restenosis.
Methods and results: The Tryton FIM study was a multicentre, prospective, single-arm, “first-in-man” study to treat de novo bifurcation lesions. Minimum lumen area (MLA) sites and overall volumes were analysed within main vessels and side branches. Overall, 27 main vessels and 22 side branches had paired baseline and follow-up IVUS. The post-intervention main vessel MLA decreased from 5.3 (4.1, 6.2) to 4.8 (3.4, 5.7) mm2 at follow-up, p=0.02, and the side branch MLA decreased from 3.5 (3.0, 3.8) to 2.5 (2.2, 3.2) mm2, p=0.0005. Stent area at the side branch did not change (mean stent area from 4.0 [3.3, 4.1] to 3.8 [3.4, 4.2] mm3/mm, p=0.95). Neointimal hyperplasia (NIH) net volume obstruction (%) measured 1.8% (0.5, 7.0) for the entire main vessel and 14.9% (2.3, 31.1) for the entire side branch stents. In both main vessel and side branches the decrease in lumen area correlated significantly with NIH.
Conclusions: Serial IVUS analysis of a new side branch Tryton stent showed no chronic stent recoil. Side branch underexpansion was common and along with superimposed NIH contributed to the reduction in lumen dimensions at follow-up.
Introduction
Bifurcation lesions are common, comprising >15% of lesions in recent “all-comer” studies. They are more difficult to treat than non-bifurcation lesions and are associated with higher acute complications and restenosis rates1-3. The bifurcation anatomy varies in terms of angle, size of the main vessel and side branch, and/or underlying plaque distribution, all of which may affect procedural outcomes. Accurate diagnosis of bifurcation lesion severity and evaluation of optimal bifurcation stent implantation are challenging. Because none of the many current interventional techniques is perfect4,5, dedicated bifurcation stents are under development6-8. The clinical and angiographic results for the Tryton FIM study have been reported previously6,9. We report the baseline and follow-up intravascular ultrasound (IVUS) findings of bifurcation lesions treated with the dedicated Tryton Side Branch Stent (Tryton Medical, Inc., Durham, NC, USA) to assess mechanisms of restenosis at the side branch ostium.
Methods
This manuscript reports the IVUS findings obtained from an analysis of the initial 45 patients treated with the Tryton Side Branch Stent (Tryton Medical, Inc.). This cohort includes the Tryton I study (initial 30 patients) and 15 additional patients who were treated in a similar fashion on a continued access basis. Tryton I was a multicentre, prospective, single-arm, “first-in-man” study designed to assess the safety and feasibility of the Tryton Side Branch Stent when used in conjunction with a standard main vessel drug-eluting stent (DES) to treat de novo, bifurcation, native coronary artery lesions5. The enrolment was done between November 2006 and May 2007. The protocol was approved by the local ethics committees, and all patients gave written informed consent.
The Tryton stent is a slotted-tube, balloon-expandable, cobalt-chromium, bare metal stent (BMS) with three zones: a distal side branch zone, a central transition zone, and a proximal main vessel zone (Figure 1). The distal side branch zone has the design characteristics of a standard slotted-tube stent. The specific central transition zone geometry contains three panels, each of which can be deformed in an independent fashion to provide complete coverage of the side branch ostium by accommodating the complete spectrum of commonly encountered bifurcating coronary artery geometry. The proximal main vessel zone has three fronds which are linked distally to the transition zone panels and terminate proximally in a circumferential band.
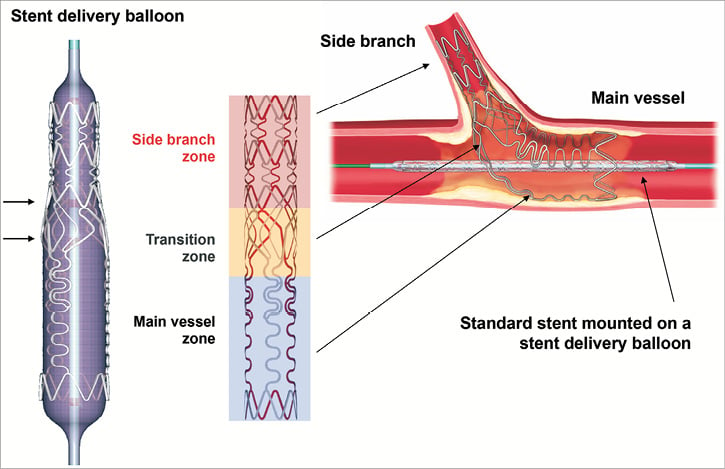
Figure 1. Schematic representing the Tryton Side Branch Stent showing three zones: distal (side branch) zone, central (transition) zone, and proximal (main vessel) zone.
The Tryton stent used in this study was 18 mm long (distal side branch zone [6 mm], central transition zone [4 mm], proximal main vessel zone [8 mm]) with a strut thickness of 0.003 inches. The available sizes of the Tryton stents were 2.5 mm for the side branch zone and either 2.5, 3.0, or 3.5 mm for the main vessel zone. The Tryton stent was mounted on either a straight balloon (uniform diameter of 2.5 mm) or a stepped balloon (proximal diameter of 3.5 mm and distal diameter of 2.5 mm). Tryton stent delivery balloons have four markers: standard proximal and distal markers and two additional markers 4 mm apart delineating the central transition zone.
The procedure was performed via a 6 Fr or larger guiding catheter. Both the main vessel and side branch were wired, and the lesion was predilated per operator’s discretion. The Tryton stent was then positioned in the side branch with the central transition zone markers straddling the side branch origin. After deployment of the Tryton stent, the stent delivery balloon was retrieved. The guidewire initially placed in the side branch was then repositioned in the distal main vessel. A standard DES was then positioned in the main vessel through the fronds of the Tryton stent across the side branch ostium into the distal main vessel. DES selection for the main vessel stent was at the operator’s discretion, but limited to TAXUS® Express2™ paclitaxel-eluting stent (Boston Scientific, Natick, MA, USA), CYPHER® sirolimus-eluting stent (Cordis, Johnson & Johnson, Warren, NJ, USA), or XIENCE V® everolimus-eluting stent (Abbott Vascular, Santa Clara, CA, USA). The side branch was re-accessed to perform final simultaneous kissing balloon inflations as was pre-specified for all cases.
Quantitative coronary analysis (QCA) was performed at an independent core lab (Cardialysis BV, Rotterdam, The Netherlands) using CASS 5.4 software (Pie Medical, Maastricht, The Netherlands)6,9. The analyst divided each bifurcation lesion into the proximal main vessel, distal main vessel, and side branch. Minimum lumen diameter, reference vessel diameter, and % diameter stenosis were analysed for each segment. The angles between the proximal main vessel and distal main vessel and between distal main vessel and the side branch were analysed. Binary restenosis was defined as ≥50% diameter stenosis within the stent or the proximal or distal 5 mm reference segments. IVUS studies were obtained at the completion of the index procedure and at six months. All IVUS studies were performed after intracoronary administration of 200 µg nitroglycerine using a commercially available IVUS system (Boston Scientific Corporation, Fremont, CA, USA, or Volcano Corporation, Rancho Cordova, CA, USA). The IVUS catheter was advanced distal to the stented segments, and imaging performed retrogradely back to the aorto-ostial junction at an automatic pullback speed at 0.5 mm/sec. Images were recorded onto digital media for off-line analysis at a single, independent core laboratory (Cardiovascular Research Foundation, New York, NY, USA) which was unaware of patient clinical outcomes.
Using computerised planimetry (echoPlaque; INDEC Systems, Mountain View, CA, USA), external elastic membrane (EEM), stent, and lumen borders were identified and manually traced. Using vascular and perivascular landmarks and the known pullback speed, baseline and follow-up images were matched and analysed side by side. EEM, stent, lumen, neointimal hyperplasia (NIH: stent minus lumen), and plaque and media (EEM minus lumen or EEM minus stent as appropriate) area were calculated every 1 mm with particular attention paid to the carina frames and minimum lumen area (MLA) sites in the main vessels and side branches10. Carina frames were chosen as the first end-diastolic frames showing “figure-of-eight” and not “snow man” shapes for both the main vessel and side branch ostia (Figure 2). Overall volumes as well as 5 mm long volumes proximal and distal to the carina were calculated using Simpson’s rule. To standardise for different overall stent lengths, mean EEM, stent, lumen, NIH, and plaque and media areas were calculated as volumes divided by length. Stent expansion was defined as minimum stent area divided by distal reference lumen area.
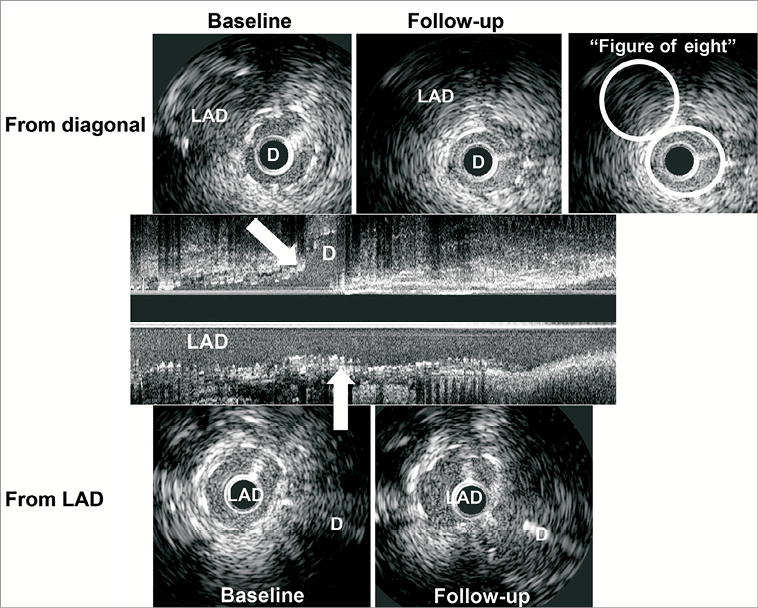
Figure 2. Representative case at carina in main and side branches. Serial IVUS images showed that stent area at carina in the LAD (baseline 4.6 mm2, follow-up 4.8 mm2) and in the diagonal branch (baseline 4.1 mm2, follow-up 4.1 mm2) were identical. The measurements at carina are in the first frame in which we can see both LAD and diagonal lumens as “figure-of-eight”, not “snow man” shape. D: diagonal; LAD: left anterior descending
Qualitative IVUS parameters included stent malapposition (blood speckle behind stent struts) categorised as persistent (visible both at baseline and follow-up), resolved (visible only at baseline), and late acquired (only visible at follow-up); intra-stent plaque and/or thrombus protrusion (IVUS cannot reliably differentiate between plaque and thrombus protruding through stent struts); stent fracture (absence of struts over more than one third of the stent circumference); aneurysm (lumen >50% larger than the proximal reference); and edge dissection.
Statistical analysis was performed using SAS software version 9.1 (SAS Institute Inc., Cary, NC, USA). Continuous variables were presented as median (Q1, Q3), and categorical variables were presented as frequencies. Continuous variables were compared using the Mann-Whitney U test. Categorical variables were compared using chi-square statistics. Correlation between baseline minimum stent area (MSA) and follow-up MLA and change of lumen area and NIH were evaluated using a linear regression model; p<0.05 was considered significant.
Results
Overall, 36 main vessels and 32 side branches had adequate baseline IVUS images, and 31 main vessels and 30 side branches had adequate follow-up IVUS images. Paired (baseline and follow-up) planar IVUS images were available for both the main vessel and the side branch in 19 cases, while paired (baseline and follow-up) planar IVUS images were available for eight patients in only the main vessel (and not the side branch) and for three patients in only the side branch (and not the main vessel). Paired volumetric IVUS analysis was available for 21 main vessels and 19 side branches. Patient characteristics, procedural, angiographic, and outcome data are shown in Table 1 both in the overall cohort (n=40) and in the paired cohort (n=30). Bifurcation in the LAD and diagonal branch is the most frequent (77.5%) and angiographic true bifurcation evaluated by operators was 70% (28/40). Predilatation of the side branch was performed in 75% of cases, and 95% of cases were finished by kissing balloon dilatation. Three patients received additional stents distal to the Tryton stent in the side branch.
Table 2 shows the QCA data both overall (n=40) and in the paired cohort (n=30). Overall, restenosis (>50% diameter stenosis at follow-up) was observed in one proximal main vessel and two side branches, and three patients underwent target lesion revascularisation (Online Figure 1). Percent diameter stenosis was comparable between the main vessel and the side branches.
In the overall cohort (Table 3) the side branch MLA site was located close to the carina (distance between carina and MLA site=1.8 mm), but the location of the main vessel MLA site was more variable (distance between carina and MLA site=5.7 mm [0.1, 12.3] with 14.8% of baseline and 20% of follow-up IVUS studies having the MLA located proximal to the carina). In the overall cohort, the post-intervention main vessel MLA measured 5.0 mm2 (4.1, 6.1), and the side branch MLA measured 3.5 mm2 (2.9, 3.9). Compared to the distal reference lumen area, stent expansion measured 85% (74, 100) in the main vessel and 77% (72, 89) in the side branches. In the overall cohort the follow-up main vessel MLA decreased to 4.8 mm2 (3.9, 5.7), and the side branch MLA decreased to 2.5 mm2 (2.1, 3.3). Percentage NIH at the MLA site measured 0% (0.0, 0.0) in the main vessel and 20.6% (0.0, 36.4) in the side branches.
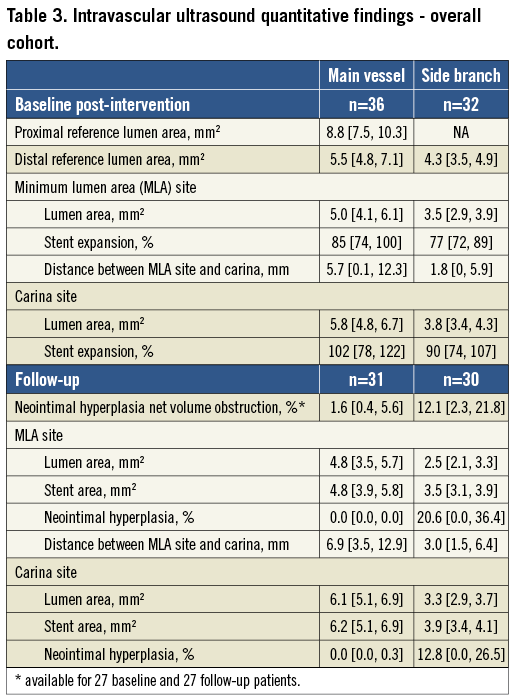
In the paired comparison (Table 4) stent area did not change whether analysed at the carina or as an entire segment distal to the carina (mean side branch stent area 4.0 [3.3, 4.1] to 3.8 [3.4, 4.2] mm3/mm, p=0.95, i.e., no chronic stent recoil). NIH net volume obstruction measured 1.8% (0.5, 7.0) for the entire main vessel and 14.9% (2.3, 31.1) for the entire side branch stents. In side branches, the decrease in lumen area significantly correlated with the NIH at the MLA site; however, the follow-up MLA did not correlate with the baseline MSA suggesting that NIH was unrelated to stent expansion. In the main vessels the decrease of lumen area was also correlated with the NIH at the MLA site; however, unlike the side branches, the majority of subjects showed little NIH so that follow-up MLA correlated with baseline MSA (Figure 3).
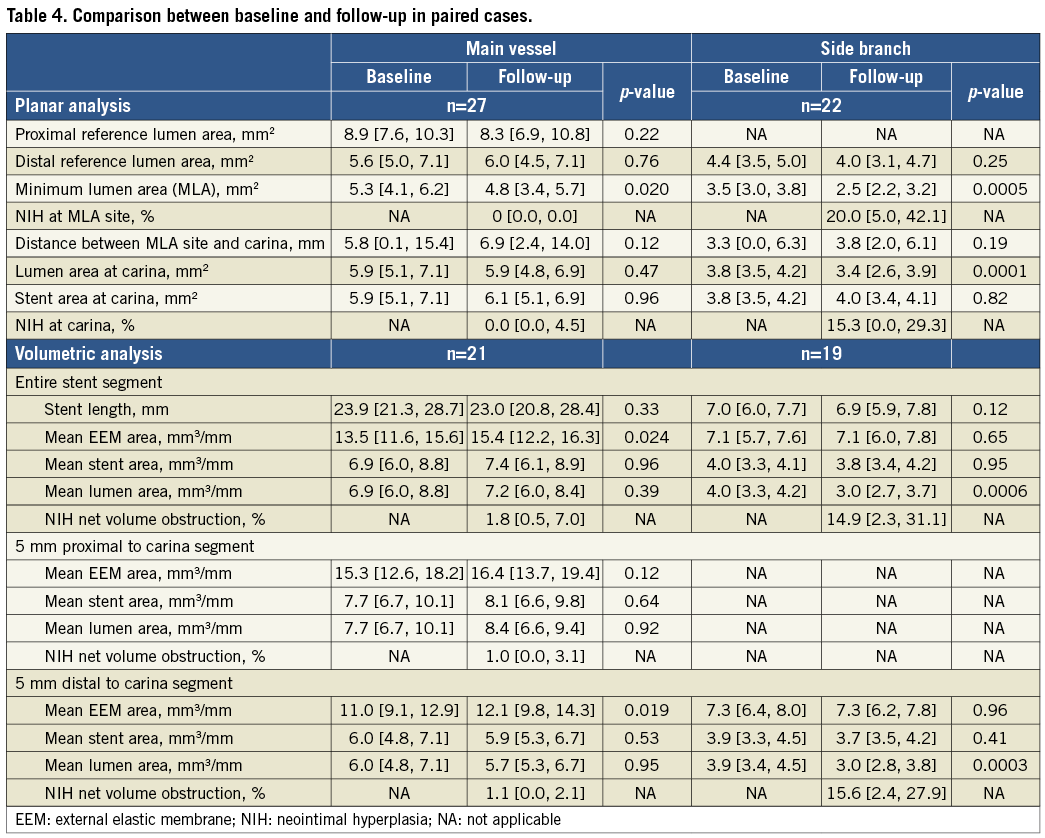
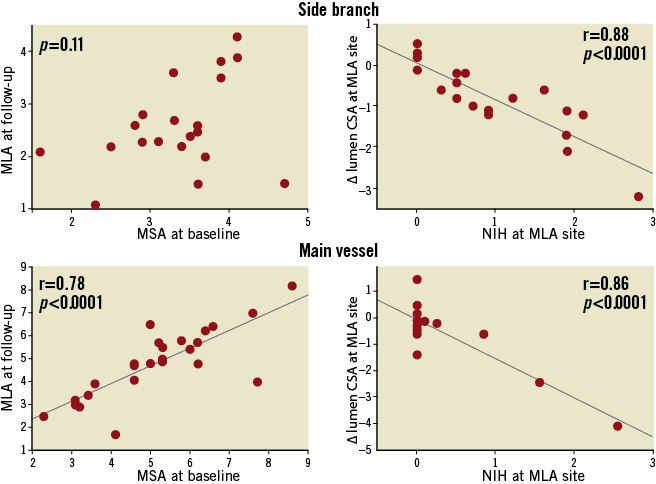
Figure 3. Correlation between post-procedural MSA and follow-up MLA and decrease of lumen area and NIH. MLA: minimum lumen area; MSA: minimum stent area; NIH: neointimal hyperplasia
Main vessels treated by DES showed positive remodelling (mean EEM CSA measured 13.5 mm3/mm [11.6, 15.6] at baseline and 15.4 mm3/mm [12.2, 16.3] at follow-up, p=0.024). However, positive remodelling was not seen in the side branches.
At baseline, acute malapposition was observed in 7/32 (21.9%) side branches (three distal edge and four stent body) and in 7/36 (19.4%) main vessels (four stent edge and three stent body) (Online Figure 2). Maximum acute malapposition area measured 0.9 mm2 (median) in the side branches and 1.2 mm2 (median) in the main vessels. There was no stent vessel wall malapposition at the bifurcation. Of the lesions included in the paired analysis, four out of five acute side branch malappositions resolved, and all seven main vessel malappositions persisted. In the paired analysis there were nine late acquired malappositions having a maximum malapposition area of 1.3 mm2 (median), all in main vessels treated with a DES and none in side branches treated with a bare metal Tryton stent. The other four malappositions were found in the main vessel: because there were no paired images, they could not be characterised as persistent or late acquired.
Post intervention, there were three medial dissections including one intramural haematoma in the side branches, and one intimal dissection and three medial dissections, including two intramural haematomas, in the main vessels. All had follow-up images, and all dissections were healed, including the intramural haematomas. Seven plaque/thrombus protrusions were seen through main vessel stents, and one plaque/thrombus protrusion was seen through the side branch Tryton stent. All disappeared at follow-up. No aneurysms or stent fractures were observed in this study.
Discussion
In this serial IVUS study of bifurcation lesions treated with a two-stent technique – the dedicated Tryton stent in the side branch and a DES in the main vessel – the main messages were as follows. 1) There was no stent recoil in either the side branch or the main vessel stent, including the side branch ostium. 2) Post-procedural side branch underexpansion was common and, along with superimposed NIH, contributed importantly to lumen dimensions at follow-up.
To date, reports of IVUS analysis for bifurcation stenting have been limited. Costa et al studied a small number (n=20) of side branch lesions treated with the crush technique: side branch stent underexpansion (MSA=3.9±1.0 mm2, stent expansion 79.9±12.3%) was similar to that observed in the current analysis (3.5 mm2 [median] MSA and stent expansion 77% [median])11. Hahn et al reported serial IVUS analysis in 73 bifurcation lesions treated by DES (CYPHER or TAXUS) T-stenting and concluded that the mechanisms of side branch restenosis were stent underexpansion (MSA; 5.0±1.0 mm2) and neointimal hyperplasia (23.8±18.9%), similar to the 20.6% (median) neointimal hyperplasia at the MLA site in the current report12. However, Hahn et al implanted DES in the side branch ostium; in the current report, the side branch ostium was treated with the bare metal Tryton stent. These findings could indicate: 1) less injury after Tryton stent implantation and/or better coverage of the side branch carina compared to conventional two-stent techniques, and/or 2) possible effect of drug elution from the main vessel DES into the carina of the side branch. In addition, NIH net volume obstruction (%) comparing the 5 mm segments proximal or distal to the main vessel carina were similarly inhibited, indicating that Tryton DES stent overlap had no impact on the antiproliferative DES effects.
Costa et al also reported a 25% incidence of incomplete crush of the side branch stents, while Hahn et al reported 4.1% acute stent vessel wall malapposition at the main vessel carina and 4.1% acute stent vessel wall malapposition at the ostium of the side branches. Acute stent malapposition in the current study did not occur at the level of the carina but within the body or edges of the stents. However, in the current study, acute malapposition within the body or edges of the stents appeared similarly in main vessels and side branches. The majority of acute side branch malappositions resolved, all main vessel acute malappositions persisted, and late acquired malapposition was observed only in the main vessel DES due to vessel positive remodelling, consistent with the previous report13,14. A similar incidence of residual edge dissection was observed in main vessels (4/36; 11.1%) and side branches (3/32; 9.4%), and all were confirmed as healed at follow-up, also similar to previous reports15,16.
To date, serial IVUS observations for two dedicated side branch or bifurcation stents have been reported. One is a self-expanding biolimus-eluting stent (Axxess™ stent; Devax Inc., Lake Forest, CA, USA) having a conical shape covering the bifurcation flaring from the parent proximal vessel into the ostia of the two daughter vessels7. The other is a self-expanding bare metal stent (Sideguard® stent; Cappella Inc., Auburndale, MA, USA) also having a funnel shape, but flaring to cover the side branch ostium and tapering into the distal side branch8. Serial IVUS analysis of the Axxess stent showed a net NIH volume obstruction of only 4.3±5.2% with a relatively high incidence of stent malapposition (acute: 35.5%, and follow-up: 27.4% including 1.6% of late acquired stent vessel wall malapposition). Serial IVUS analysis of the Sideguard stent showed an increase of stent area at the carina (3.9±1.2 mm2 at baseline to 4.6±1.1 mm2 at follow-up) resulting in preserved lumen dimensions despite NIH accumulation. The number of cases, especially paired cases, was small. Three types of drug-eluting stent were used for main vessel stenting.
Study limitations
The number of cases, especially paired cases, was small. Three types of drug-eluting stent, CYPHER, TAXUS, and XIENCE, were used for main vessel stenting. Because this was a first-in-man study, aggressive post-dilation was not recommended. Therefore, the prevalence of underexpansion may not reflect the real world. Incomplete paired baseline and follow-up imaging in one of the two branches, most commonly due to failure to advance the IVUS catheter into one of the two distal vessels, may have introduced bias into the analysis.
Conclusions
Serial IVUS analysis of a two-stent strategy using the new side branch Tryton stent showed no chronic stent recoil. However, post-procedural side branch underexpansion was common and, along with superimposed NIH, contributed importantly to lumen dimensions at follow-up.
| Impact on daily practice The major findings in the present serial IVUS observations from the Tryton first-in-man study were as follows: 1) there was no stent recoil in either the side branch or the main vessel stent, including the side branch ostium; 2) post-procedural side branch underexpansion was common and, along with superimposed neointimal hyperplasia, contributed to lumen dimensions at follow-up. In daily practice, the recognition of acute stent underexpansion and optimisation by IVUS will be useful to prevent restenosis after treating a bifurcation lesion with a dedicated stent such as Tryton. |
Guest Editor
This paper was guest edited by Fernando Alfonso, MD, PhD, Interventional Cardiology, Cardiovascular Institute, IdISSC, Clínico San Carlos University Hospital, Madrid, Spain.
Conflict of interest statement
A. Maehara and G.S. Mintz report receiving research grants from Boston Scientific (Fremont, CA, USA). H.R. Davis is an employee of Tryton Medical, Inc. A.V. Kaplan is the founder and a board member of Tryton Medical, Inc. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.
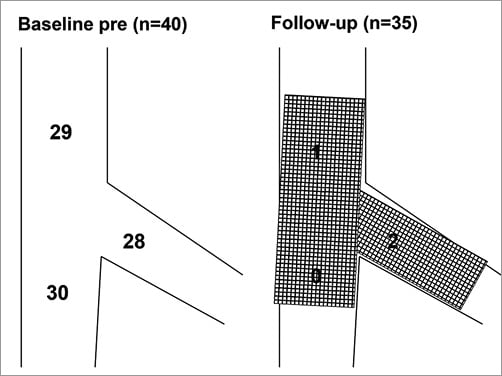
Online Figure 1. Angiographic lesion location and restenosis location at follow-up. Lesion location by Medina classification was summed for each proximal main vessel (29 stenoses), distal main vessel (30 stenoses), and side branch (28 stenoses) in 40 patients. At follow-up, among patients with available follow-up, angiography showed one restenosis in the proximal main vessel and two restenoses in the side branch.
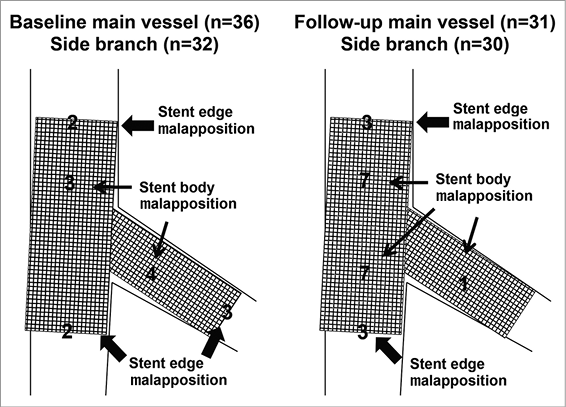
Online Figure 2. Malapposition location at baseline and follow-up. At baseline, there were two malappositions at the proximal edge of the main vessel stent, two malappositions at the distal edge of the main vessel stent, three malappositions in the proximal body of the main vessel stent, four malappositions in the body of the side branch stent, and three malappositions at the distal edge of the side branch stent. At follow-up, there were three malappositions at the proximal edge of the main vessel stent, three malappositions at the distal edge of the main vessel stent, seven malappositions in the proximal body of the main vessel stent, seven malappositions in the distal body of the main vessel stent, and one malapposition in the body of the side branch stent.

