Abstract
Aims: Dedicated bifurcation stents should facilitate deployment and improve coverage of bifurcational lesions. We used optical coherence tomography (OCT) to assess bifurcation lesions treated with a dedicated stent implanted in the side branch (SB) in conjunction with drug eluting stents in the main vessel (MV) in a culotte-like fashion.
Methods and results: Nine patients treated with the Tryton stent underwent postprocedural OCT examination. Total percent of malapposed struts per patient was 18.1±8.7%. The longitudinal distribution of the percent of malapposed struts per patient showed that the prevalence of malapposed struts was significantly higher at the level of the bifurcation (33.3%), than in both the proximal segment and the distal segment (18.5% and 9.8%, respectively, p=0.011). When the bifurcation was divided into two halves (opposite SB and toward SB), the highest percent of malapposed struts was toward the SB (47.6%). Also the wall-strut distance for malapposed struts was significantly higher in the bifurcation half toward the SB than in the proximal and the distal segment.
Conclusions: Malapposed struts are frequent in bifurcations despite the use of a dedicated stent. The highest frequency and largest vessel wall–stent strut distance are observed in the bifurcation half toward the SB.
Abbreviation list
DES: drug eluting stent
IQR: interquartile range
IVUS: intravascular ultrasound
KB: kissing balloon
MLA: minimal lumen area
MV: main vessel
OCT: optical coherence tomography
QVA: Quantitative vascular arteriography
SB: side branch
SD: standard deviation
UFH: unfractionated heparin
Introduction
Drug eluting stents (DES) reduce restenosis in coronary bifurcations1, but concerns remain regarding a higher incidence of stent thrombosis2 which may be explained by the increased prevalence of malapposed stent struts3. When intimal hyperplasia is negated by a powerful antiproliferative coating, malapposition may persist for years after implantation and create an increased risk of late stent thrombosis4. In principle, a single stent approach does not provide as good a scaffolding for the whole bifurcation as other techniques of double stenting. However, when universal double stenting has been compared with a single stent approach in randomised trials, results have been neutral or in favour of single stenting1,5-7.
Intravascular ultrasound (IVUS) analyses have specifically excluded bifurcation segments from the assessment of incomplete stent apposition8,9, because the prominent artifacts generated by the stent struts and the low resolution of the technique preclude detailed assessment of the complex geometry of a bifurcation. Optical coherence tomography (OCT), due to its high spatial resolution, allows accurate evaluation of strut apposition and assessment of the ostium of the bifurcation during a single pull-back in the main vessel (MV)10.
The Tryton-Side Branch Stent™ (Tryton Medical, Inc., Newton, MA, USA) is a dedicated bifurcation stent (Figure 1), designed to be implanted in the side branch (SB) of bifurcations in conjunction with a conventional DES in the MV in a culotte-like fashion11. Because of large openings in the transition zone, and a proximal segment with few sparse struts designed to fix it in place proximally, the Tryton stent has the potential to reduce malapposition.
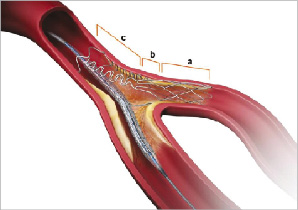
Figure 1. Tryton stent. There are three zones: a- side branch, b- transition, c- main vessel.
The aim of this study was to assess strut apposition by OCT in a bifurcation lesions treated by implantation of a Tryton stent using a modified culotte technique.
Methods
Study population
All consecutive patients, who underwent postprocedural OCT examination after Tryton stent implantation for the treatment of bifurcational lesions between March 2007 and October 2008 were included in the study (n=9).
Pharmacological treatment and procedural devices
Before the procedure, all patients were pretreated with aspirin and 300 or 600 mg of clopidogrel. During the procedure unfractionated heparin (UFH) or bivalirudin was used: UFH was given to maintain an activated clotting time ≥250 seconds with an initial bolus of 70 IU/kg, whilst bivalirudin was given according to the patient’s body weight. Administration of glycoprotein IIb/IIIa inhibitors was at the operator’s discretion. In all cases, 6 Fr guiding catheters were used. High pressure or cutting balloon predilatation as well as postdilatation with a high pressure balloon and kissing balloon (KB) postdilatation were performed in all cases.
Stent design and implantation
The Tryton Side-Branch Stent™ is a cobalt-chromium stent designed for bifurcation lesions11. It consist of three zones: I-side branch (length: 6 mm), II-transition (length: 4 mm), III-main vessel (length: 8 mm). There are two stent delivery systems. In all the cases except for one we used the stepped balloon system, which has an inflated geometry that corresponds to the three Tryton stent zones with following distal/proximal diameters: 2.5/3.5; 3.0/3.5; 3.0/4.0; 3.5/4.0 mm at nominal inflation pressure11. The strut thickness is 0.0033” (84 µm).
The Tryton stent was implanted using a modified culotte technique: two guidewires are advanced into the SB and the MV. The Tryton stent is then introduced into the SB and subsequently aligned using transition zone markers (Figure 2) and deployed. After withdrawal of the stent delivery balloon, the guidewire is switched from the SB into MV without withdrawing it behind the connecting ring in the MV in order to avoid crossing between the connecting ring and MV wall. The second stent (always a drug eluting stent in this series) is then advanced into the distal MV through the wide space between the proximal fronds of the Tryton stent followed by rewiring and final KB. The first successful implantation of this stent with post-procedural OCT examination has recently been reported12.
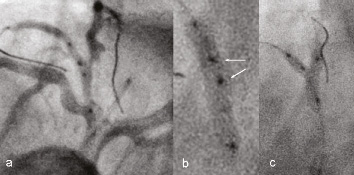
Figure 2. a) Positioning of the Tryton stent. There are two visible points of the transition zone in the middle part of the stent (arrows on Figure b), at the level of the SB ostium, b) Tryton stent during deployment, c) Kissing balloon post-dilatation.
Quantitative angiographic analysis
All bifurcation lesions were classified according to the Medina classification13.
Quantitative vascular arteriography (QVA) was performed using dedicated software (QAngio XA 7.1, Medis Medical Imaging System, Leiden, The Netherlands), as previously described14.
Bifurcation angles were measured off-line using the CardiOp-B® software package (Paeion Inc, New York, NY, USA). This system enables 3D reconstruction of coronary arteries using two or three standard angiographic views, provided that they are at least 30° apart (Figure 3c). Bifurcation angle measurement included both the angle between the proximal MV and the SB as well as the angle between the distal MV and the SB and was possible in all nine cases.
OCT imaging technique
In this study, an end-hole microcatheter (0.021” Transit™, Cordis Neurovascular, Miami Lakes, FL, USA) was advanced distal to the lesion in the MV over a conventional guidewire, which was then exchanged for the OCT imaging wire. OCT image acquisition (LightLab Imaging Inc. Westford, MA, USA) was performed using a non-occlusive technique15 with continuous flushing using a power injector (2-5 ml/sec) of iodixanol (Visipaque™, GE Healthcare, UK) at a pullback speed set at 3 mm/sec. Image acquisition over 30 to 35 mm vessel segments was performed without complication.
OCT image analysis
Cross-sectional images were analysed every 450 microns. Since the strut alloy is opaque to infrared light, stent struts were defined as malapposed if the distance between the luminal surface of the strut and the vessel wall was greater than the thickness of the strut (metal + polymer) plus an additional 15 microns (because the OCT spatial resolution is 10-20 microns)16. The thickness of stents used in the study are as follows: Cypher Select 154 µm, Taxus Liberte 127 µm, Endeavor Resolute 95 µm, Xience V 88 µm16. Strut apposition was assessed in three segments: proximal MV segment (extending up to 8 mm from the first cross-section when the SB is visible), bifurcation (divided into two 180 degrees halves towards or opposite the origin of the SB) (Figure 4), and distal MV segment (extending up to 4 mm from the last cross-section when the SB is visible) (Figures 3-6). At the level of bifurcation and in the proximal segment there are two different types of stents with different strut thickness- the MV stent and the SB Tryton stent. Since it is impossible to distinguish if malapposed struts (especially at the bifurcation level) are from the MV stent or from the Tryton stent, strut malapposition was calculated on the basis of the MV stent type and strut thickness. The number of fronds of the Tryton stent in the transition zone is minimised and in the proximal part is limited only to three. Secondly, the Tryton stent is the outer stent and the inner stent is the MV stent. That means that the most likely the malapposed struts are from the MV stent. All distances were measured in perpendicular cross-sections from an OCT pullback in the MV.
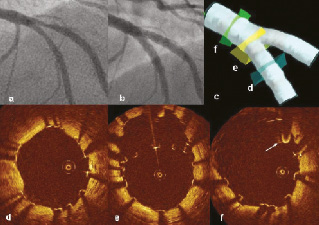
Figure 3. a) Bifurcation lesion involving the left anterior descending artery and diagonal branch (Medina classification: 0,1,1), b) Angiographic result after deployment of a Tryton stent in the SB and DES in the MV, c) Artificial reconstruction of the bifurcation by CardiOp-B® software with corresponding OCT images (d-f). OCT cross sections: d) Distal MV- there are visible single malapposed struts; e) Bifurcation just proximal to carina- malapposed struts creating a metallic neo-carina; f) Proximal MV- there are visible single malapposed struts and guidewire (arrow).
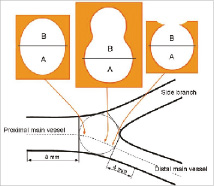
Figure 4. The scheme representing sequential cross sections of the bifurcation divided into two halves: A- the half opposite SB, B- the half toward SB. In the proximal MV strut apposition was assessed up to 8 mm before the bifurcation and in the distal MV up to 4 mm beyond the bifurcation.
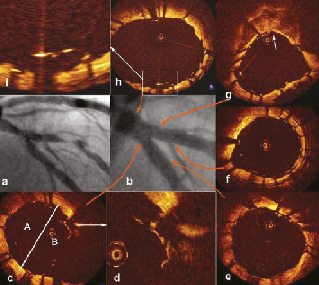
Figure 5. a) Bifurcation lesion involving the left anterior descending artery and diagonal branch, b) Angiographic result after deployment of a Tryton stent in the SB and DES in the MV. Pullback from the MV and from the SB. OCT cross sections: c) Bifurcation- visible malapposed struts in the half facing the SB (A- the half opposite SB; B- the half toward SB), d) Magnification of picture c focused on malapposed struts, e) Distal segment of the MV- well apposed struts, f) Distal segment of the SB- malapposed struts at the level of small branch; g) OCT image immediately distal to SB ostium shows irregular contours of the vessel lumen and confirms the presence of compressed plaque behind struts (arrow), which is typically located opposite the SB, h) Proximal MV- most struts are well apposed and only a few are malapposed, g) Magnification of image h focused on malapposed struts.
Cardiac biomarkers
Troponin I was measured routinely 12 to 24 hours after the procedure. Elevation of ≥ 3 times the upper limit of normal (0.04 µg/L) was considered significant and defined as periprocedural MI (with or without pathological Q waves), in the absence of another aetiology.
Statistics
Continuous variables are expressed as mean±standard deviation (SD) or median and interquartile range (IQR). Differences among four segments were assessed with ANOVA or Kruskal-Wallis test. The comparison between two groups was performed with unpaired t test or Mann Whitney U-test; Bonferroni’s correction was used for multiple comparisons (p value <0.0083, 0.05/6 was the level of statistical significance).
To assess whether the strut location in the bifurcation half toward SB may increase the risk of strut malapposition and may affect the stent strut-vessel separation distance, we performed a multilevel mixed effect logistic regression and multilevel mixed effect linear regression, respectively, fitting a model with three levels. This model accounts for the correlated nature of the data and allows for the heteroscedasticity of the random effects: level 1=the single strut, level 2=the segment (proximal, distal, bifurcation half toward SB and opposite SB), level 3=the patient, adding the presence/absence of malapposed struts toward SB location as random effect at the level 1 and level 2. The statistical significance was a p value <0.05. Statistical calculations were performed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA) and STATA 10.1 statistical software (StataCorp LP, College Station, TX, USA).
Results
Sixteen patients were treated with the Tryton culotte technique, nine of whom had additional postprocedural OCT examination. Table 1 summarises demographical and clinical data: 56% of the patients were male with an average age of 66.7±7 years; 44% of patients had two or three vessel disease.
Table 2 shows angiographic and procedural details. The target bifurcation lesion was the left anterior descending artery/diagonal branch in seven patients and the left circumflex artery/obtuse marginal branch in two patients. Significant ostial SB stenosis (>50% diameter stenosis) was present in seven lesions. The following DES were implanted into the MV: Cypher Select (n=4), Taxus Liberte (n=2), Endeavor Resolute (n=1) and Xience V (n=2). Final KB was performed in all nine cases. Procedural success by angiography with TIMI 3 flow was achieved in all lesions. In all cases, a mild post-procedural troponin I rise in routine blood tests was noticed (median 0.58 µg/L, IQR 3.41), including one patient with troponin I rise up to 20 µg/L due to occlusion of a small septal branch. No ischaemic ECG changes or pathological Q waves were observed. No other major adverse cardio-vascular events occurred during the in-hospital stay.

The OCT analysis is presented in Table 3 and 4. A total of 2,782 struts were analysed: 1,514 (54.5%) struts in the proximal vessel segment, 449 (16.1%) struts at the bifurcation level and 819 (29.4%) struts in the distal vessel segment. The total number of malapposed struts was 494: 243 in the proximal segment, 133 in the bifurcation and 118 in the distal segment. The overall total percent of malapposed struts was 17.8%, and the mean total percent of malapposed struts per patient was 18.1±8.7%. Per patient analysis of the longitudinal distribution of malapposition showed that the prevalence of malapposed struts was higher in the bifurcation half toward SB (47.6% [35.3-58.6]) than in the distal segment (9.8% [4.5-14.3], p=0.0023), the proximal segment (18.5% [10.5-21.1], p=0.0054), and the bifurcation half opposite SB (14.8% [12.9-19.5], p=0.0054), with no significant difference between proximal and distal segments (p=0.27), bifurcation half opposite SB and proximal segment (p=0.96), bifurcation half opposite SB and distal segment (p=0.35), (p=0.0035 among the four groups). At multilevel mixed effect logistic regression, the location of the struts toward SB carried a significant striking increased risk of malapposition (odds ratio [OR] 5.8, 95% confidence interval [CI] 3.2 -10.7, p<0.001).
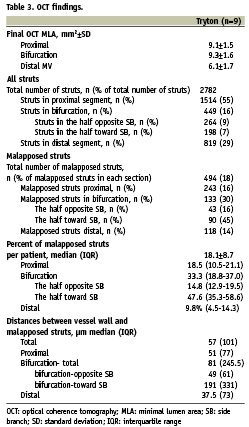
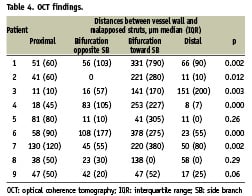
Also the strut- vessel wall distance for malapposed struts was higher in the bifurcation half toward the SB (191 μm, [40-371]) compared to the bifurcation half opposite SB (49 μm, [20-80], p=0.0001), the proximal segment (51 μm, [21-98], p<0.0001) and distal segment (37.5 μm [17-90], p<0.0001) with no significant difference between proximal and distal segments (p=0.14), bifurcation half opposite SB and proximal segment (p=0.48), bifurcation half opposite SB and distal segment (0.77), (p=0.0001 for the comparison among the four groups). At multilevel mixed effect linear regression, the location of the struts in the bifurcation half toward SB increased the strut vessel wall separation distance (coefficient 174.8 µm, 95% CI [85.8- 263.9], p<0.001).No significant correlation was observed between the percent of malapposed struts (including the analysis of the total number of malapposed struts and the analysis of the individual vessel segments: proximal, bifurcation, distal) and the bifurcation angles (both the angle between the proximal MV and the SB as well as the angle between the distal MV and the SB).
Discussion
Two theoretical advantages could be expected from using the Tryton stent for the treatment of bifurcation lesions. First, overlapping of struts in the MV, which by itself carries an increased rate of malapposition16, is minimised by the stent design. Secondly, the large cell size in the transition zone facilitates deployment and optimises alignment of the stent placed in the MV. The results of this study showed that malapposition remained relatively high at the bifurcation level despite routine high pressure postdilatation and KB dilatation with balloons of matching diameter to the distal MV and SB segments. The geometry of a bifurcation is very different from the configuration assumed by two KB, especially when inflated at high pressure: the distal segment of the balloons in the SB and MV, if appropriately sized, are likely to achieve similar apposition to that in straight vessel segments. At the origins of the SB, however, the diameters of the elliptical cross-section of the lumen are larger than the circular diameter of the proximal SB, a phenomenon more evident when the angle between the MV and daughter vessel is acute. This may leave one or more rows of malapposed struts proximal, distal or on both sides dependent on the site at which the wire has crossed the deployed MV stent to re-enter the SB lumen. In the proximal MV, the elliptical geometry of the two balloons may stretch the wall in one direction and generate malapposition in the other (Figure 5h). The ideal method to prevent malapposition in the proximal MV segment is to use a single balloon, the diameter of which is matched to the vessel segment immediately proximal to the SB origin. The highest rate of malapposed struts and the highest distance between malapposed struts and the vessel wall were at the level of the bifurcation, facing the SB. Malapposed struts in this segment often create a metallic neo-carina (Figure 6), which is also observed in other techniques for bifurcation treatment3. As the main force vector of expanding KB dilatation is axial and not longitudinal, clustering of malapposed struts in a new carina is almost inevitable. The percent of malapposed struts observed in this study (18.1% in total; 30.1% in bifurcation) must be compared with existing OCT data in bifurcations and overlapping segments. In previous OCT observations, the rate of malapposed struts following the treatment of simple lesions in straight vessel segments was 9%17, while the rate of malapposed struts in overlapping stents was as high as 41.8%, compared with 20.1% and 9.7% in non-overlapping proximal and distal segments, respectively16. It is unclear whether immediate strut malapposition in overlapping segments will remain present at follow-up in the majority of DES. Consistent with previous OCT observations18, the ODESSA trial showed that the highest rate of strut malapposition observed at six months was in overlapping DES segments as compared to non-overlapping segments (2.6% vs. 0.8%, respectively), and the stents characterised by the highest rates of malapposed struts were Taxus (5.5%) and Cypher (2.9%)19. However, the rate of malapposed struts observed immediately after stent implantation may decrease over time and our post-implantation results are consistent with previous post-implantation findings16,17. Finally, the clinical presentation of patients could potentially influence the rate of strut malapposition by influencing the decision to use a complex two stent technique20. The resistant ostial stenoses of bifurcations (often highly fibrotic or calcific), require high pressure expansion with low compliance short balloons. This is performed before the final kissing, with balloon sizes and pressures higher than the balloons of the final KB as both provisional stenting may cause carina displacement21 and deployment of stent in a second branch may lead to distortion of the first stent22. Final KB, which in this series was performed in 100% of cases, seems to be essential. Better long-term clinical results after KB are supported by previous observations. Adriaenssens et al reported that in their series of 134 bifurcation lesions in 132 patients treated with culotte technique, final KB tended to have a protective effect against stent thrombosis23. Also, after the “crush” technique, final KB significantly reduced the SB late lumen loss at nine months angiographic follow-up24. In all modern, bifurcation trials (BBC ONE trial10, CACTUS trial25), KB after complex two stent techniques was mandatory.
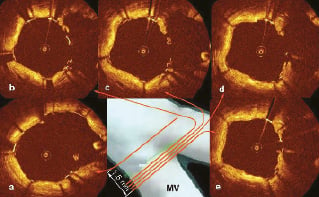
Figure 6. Creation of metallic neo-carina. a) Well apposed struts in proximal part of the bifurcation (arrow- guidewire), b-e) Sequential OCT cross sections show metallic neo-carina.
Limitations
Since we did not compare the Tryton stent to other bifurcation stents or stenting techniques, we are unable to state whether this new stent is better or worse than expected. Furthermore, the limited number of patients meant that the influence of patient and angiographic data could not be correlated to the number of malapposed stent struts. It is also possible that the use of different drug-eluting stents may have affected our findings.
As in any angiographic study, in which a three-dimensional structure is assessed by two-dimensional projection imaging, the results of measurement are influenced by the imaging angle.
Because of the small number of patients included in the study, statistical analysis was calculated with simple parametrical and non-parametrical tests.
Conclusions
OCT assessment of stent apposition after the treatment of bifurcation lesions with a new dedicated bifurcation stent in the SB and DES in the MV in a culotte-like fashion showed non-uniform distribution of malapposed struts. The highest percent of malapposed struts and the largest vessel wall–stent strut distances were observed in the bifurcation half facing the SB. No difference was observed between the rate of malapposed struts in the proximal segment, the bifurcation half opposite the SB and the distal segment.

