Abstract
Aims: Tryton side branch (SB) reverse culotte stenting has been employed for the treatment of left main (LM) stem bifurcations in patients at high risk for bypass surgery. The aim of this study was to assess acute angiographic results and six-month clinical outcome after implantation of the Tryton stent in the LM.
Methods and results: We studied 52 consecutive patients with LM disease treated in nine European centres. Angiographic and clinical data analysis was performed centrally. Fifty-one of 52 patients (age 68±11 yrs, 75% male, 42% unstable angina, SYNTAX score 20±8) were successfully treated with the Tryton stent. Medina class was 1,1,1 in 33 (63%), 1,0,1 in 7 (13%), 1,1,0 in 3 (6%), 0,1,1 in 8 (4%) and 0,0,1 in 1 (2%). The Tryton stent on a stepped balloon (diameter 3.5-2.5 mm) was used in 41/51 (80%) of cases. The mean main vessel stent diameter was 3.4±0.4 mm with an everolimus-eluting stent employed in 30/51 (59%) of cases. Final kissing balloon dilatation was performed in 48/51 (94%). Acute gain was 1.52±0.86 mm in the LM and 0.92±0.47 mm in the SB. The angiographic success rate was 100%; the procedural success rate reached 94%. Periprocedural MI occurred in three patients. At six-month follow-up, the TLR rate was 12%, MI 10% and cardiac death 2%. The hierarchical MACE rate at six months was 22%. No cases of definite stent thrombosis occurred.
Conclusions: The use of the Tryton stent for treatment of LM bifurcation disease in combination with a conventional drug-eluting stent is feasible and achieves an optimal angiographic result. Safety of the procedure and six-month outcome are acceptable in this high-risk lesion PCI. Further safety and efficacy studies with long-term outcome assessment of this strategy are warranted.
Introduction
Stenting of the left main (LM) stem is increasingly recognised as a valid revascularisation strategy in patients with significant disease involving this important segment of the coronary tree. Although coronary artery bypass surgery (CABG) is considered a superior treatment option for LM disease, some patient subgroups may still benefit from revascularisation by PCI. Randomised trials of LM PCI versus CABG have repeatedly revealed target vessel revascularisation (TVR) as the Achilles heel of the former1. However, recent studies have shown that patients with less diffuse coronary disease in low SYNTAX score tertiles have comparable outcomes with PCI and CABG2. Having secured at least a similar safety profile, PCI of LM stenosis has been upgraded to a class IIa or IIb indication in the current European Society of Cardiology/European Association for Cardio-Thoracic Surgery and American College of Cardiology/American Heart Association practice guidelines3,4.
Not all LM lesions have similar outcomes and, in fact, involvement of the LM bifurcation is associated with suboptimal short-term and long-term results when conventional stents are employed. Poor angiographic results are often obtained with the use of a provisional stenting technique in cases where the side branch (SB) is significantly diseased. Refinement of interventional techniques and the introduction of dedicated devices may improve the outcome of LM PCI. The use of a dedicated bifurcation stent –the Tryton side branch stent in conjunction with a conventional stent in a “reverse culotte” technique– facilitates stenting and may potentially improve angiographic results and clinical outcome in patients who undergo LM bifurcation intervention. Indeed, use of this dedicated stent has been increasing in the “real world” even in “off-label” anatomic locations, including the LM bifurcation5.
The aim of the present study was to assess the acute angiographic outcome of LM bifurcation treatment with the Tryton stent (Tryton Medical, Inc., Durham, NC, USA) in conjunction with a conventional stent. The acute and mid-term clinical outcome up to six months was also investigated.
Methods
The Tryton LM registry was established by retrospective inclusion of patients treated in nine European centres by very experienced operators. Procedural and clinical data of all consecutive patients in whom treatment of the LM with a Tryton stent was attempted were collected on a purposely designed electronic case report form (CRF), which was sent to the investigators. The investigators at each centre (see authors) were responsible for filling in the forms and vouch for the integrity of the data provided centrally for analysis. Angiography films of the procedures and follow-up angiograms (when available) were collected centrally and analysed by three experienced interventional cardiologists (MM, CG, RJvG).
In this report, we present the data of the first 52 patients with LM bifurcation disease in whom the operators intended to use the Tryton stenting technique. The inclusion period spanned May 2008 to October 2011.
Prespecified primary endpoints of the study included acute gain in the three segments of the bifurcation as measured by quantitative coronary angiography (QCA) as well as procedural and six-month clinical outcome in terms of major adverse cardiac events (MACE).
Patient population
Patients included were selected by the operators and, whenever feasible, after case discussion by a heart team. In general, patients were either unfit for surgery, had previous bypass surgery, had isolated LM with low SYNTAX scores or presented as an emergency. The size of the Tryton stent available at the time of patient enrolment limited the range of vessel sizes that could be treated. The visually estimated reference diameter of the main vessel (MV) could be 2.5-5.0 mm and that of the SB in the range 2.5-2.75 mm. Also, the LM had to be ≥7 mm in length to allow an adequate landing zone of the proximal part of the stent. However, the decision to treat the bifurcation and employ a Tryton stent remained at the discretion of the treating interventional cardiologist.
Device description and deployment sequence
The Tryton stent is a balloon-expandable cobalt chromium stent with strut thickness of 84 µm. The stent is available in one standard length of 19 mm and has three distinct zones: a distal SB zone, which is 6.5 mm long, a central transition zone 4.5 mm in length, and a proximal MV zone of 8 mm. The distal zone has a standard slotted tube workhorse stent design; the central transition zone consists of three panels, while the proximal MV zone is composed of three fronds that terminate proximally in two circumferential bands (only one in the first-generation device). The stent is mounted either on a balloon with uniform diameter (straight type) or on a stepped balloon (tapered type). Stent sizes (proximal-distal) available during the study period were 2.5-2.5; 3.0-2.5; 3.5-2.5 and 3.5-3.0. The stent delivery system has four markers to delineate the proximal and distal ends of the stent as well as the proximal and distal parts of the transition zone. After optional wiring of both MV and SB for predilation, the Tryton stent is advanced over the wire into the SB and is positioned with the guidance of the two middle markers which should straddle the carina. Deployment of the stent is followed by retraction of the guidewire from the SB and repositioning it through the fronds of the transition zone into the distal MV. A standard stent is then advanced, positioned, and deployed in the MV, jailing the stented SB. Proximal optimisation technique can be employed at this time by inflating a balloon the size of the proximal MV with the distal marker at the ostium of the SB. This may facilitate wire recross into the SB for final kissing balloon (FKB) dilation. A case example is presented in Figure 1. Further details of the Tryton stent and deployment procedure have been described in detail elsewhere6.
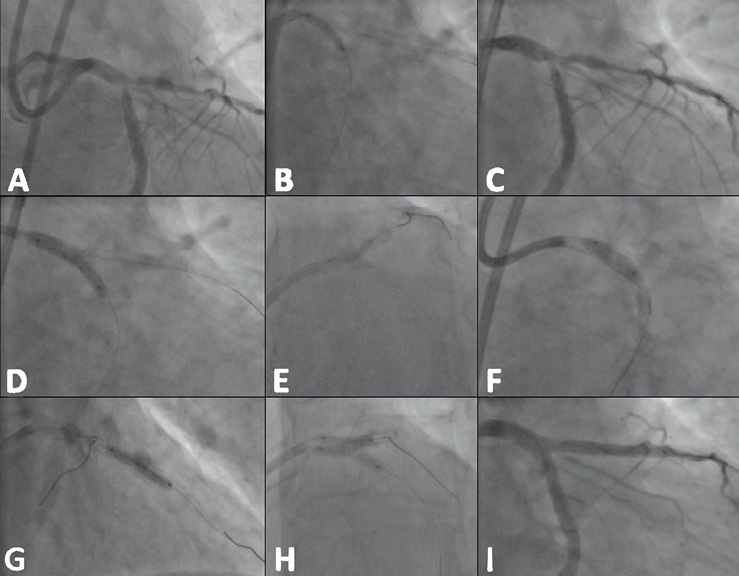
Figure 1. Implantation sequence of the Tryton stent in the left main stem bifurcation. This 78-year-old man was deemed high risk for surgery when he presented with unstable angina pectoris. The left main (LM) stem showed a Medina class 1,1,1 bifurcation lesion and further disease in segment 6 (A). The left anterior descending (LAD) and left circumflex (LCx) coronary arteries were wired and the latter was predilated with a 2.5 mm balloon (B). A 3.5-2.5×19 mm Tryton stent was positioned with the middle markers straddling the carina (C) and subsequently implanted by inflating the stepped balloon (D). The LCx wire was then retracted to the point of bifurcation and redirected into the LAD. After removal of the jailed first LAD wire, the LAD was predilated with a balloon (E) and a 3.5×18 mm XIENCE PRIME™ stent (Abbott Vascular, Redwood City, CA, USA) was placed along the LAD and across the LCx (F). After implantation of the stent, a second overlapping stent was used to treat the stenosis in segment 6 (G). The LCx was then rewired and final kissing balloon inflations were performed (H) with a good angiographic result (I).
Procedure
Patients were pretreated with aspirin and clopidogrel. Intravenous heparin was administered to maintain an activated clotting time of >250 seconds. Glycoprotein IIb/IIIa inhibitor use was left to the discretion of the treating interventional cardiologist. Delivery failures, need for additional overlapping stents to cover the whole lesion, additional balloon dilation and procedural angiographic and clinical complications were noted. Aspirin was prescribed indefinitely while clopidogrel was prescribed for at least 12 months after the index procedure.
Clinical follow-up
In-hospital events were recorded in the patient’s clinical notes. Clinical follow-up was obtained by clinical visits or phone interview during which data on hospital readmission and MACE were collected. Medical records, discharge summaries and any repeat angiography films of patients with suspected events were systematically reviewed and adjudicated by local cardiologists according to established criteria defined below.
Definitions
Device success was defined as successful deployment of the intended stent without system failure. Angiographic success was defined as <30% residual stenosis and TIMI 3 flow in both MV and SB after the procedure. Procedure success included angiographic success in the absence of in-hospital MACE, defined as a composite of cardiac death, myocardial infarction (MI) and ischaemia-driven TVR. MI was defined as clinical signs of acute ischaemia, associated with a CK-MB mass or troponin-T/troponin-I increase to ≥3 times the upper limit of normal and corresponding changes on a 12-lead electrocardiogram (ECG). TVR was defined as revascularisation of any part of the index coronary artery. Definite stent thrombosis was defined according to the Academic Research Consortium (ARC) definitions7.
Angiographic analysis
QUANTITATIVE CORONARY ANGIOGRAPHY
QCA was performed with the Cardiovascular Angiography Analysis System (CAAS; Pie Medical Imaging, Maastricht, The Netherlands) version 5.10, by experienced analysts blinded to the clinical data. All available studies were analysed with a dedicated 2-D bifurcation QCA algorithm, using matched projections, pre- and post-procedure, and end-diastolic frames. The vessel distal to the LM bifurcation where the distal Tryton stent was implanted was defined as the SB, whereas the vessel where the conventional stent was implanted was identified as the distal main vessel (DMV). A device-oriented analysis was performed, whereby the region of interest around the LM bifurcation was demarcated according to the stent borders as identified on the positioning/implantation cine runs. Specifically, the Tryton stent proximal and distal edges were defined as the proximal border of the proximal main vessel (PMV) and the distal SB border, respectively, whereas the distal DMV border was set at either the distal edge of the DES implanted through the Tryton stent or at the first major SB distal to the LM bifurcation in case of multiple overlapping stents. The minimal lumen diameter (MLD), reference vessel diameter (RVD) and percent diameter stenosis (DS) were quantified for the stented vessel segments and 5 mm peri-stent segments. Respective values for corresponding stent and segment were obtained from the six-segment bifurcation model (segments 1 and 2 for PMV, 3 and 4 for DMV, 5 and 6 for SB)8; in addition, quantitative parameters for 3 mm ostial segments were derived from the 11-segment bifurcation model (segment 11 and 8 for DMV and SB, respectively) (Figure 2 and Figure 3). Reference vessel obstruction analysis was performed with either the automatic (interpolation) method or local reference method (single point for each vessel segment). The second method was mostly reserved for diffusely diseased vessel segments prior to treatment9. Finally, proximal and distal bifurcation angle (BA) values were calculated.
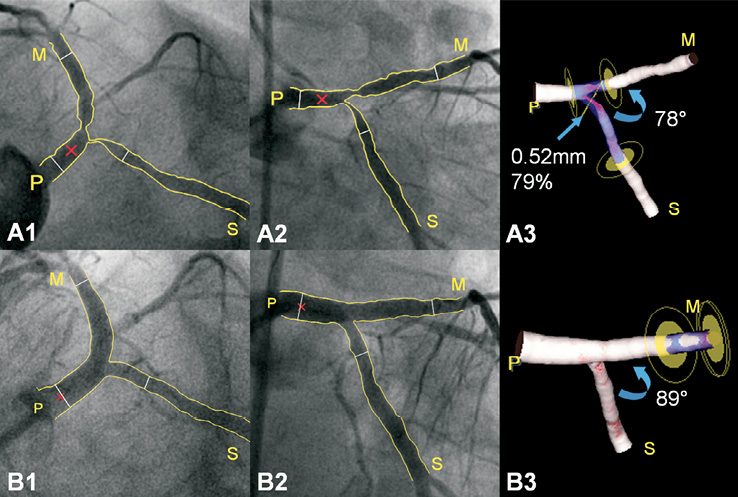
Figure 2. Three-dimensional reconstruction pre- and post-procedure. A1-A2: A left and a right anterior oblique caudal view of a left main (0,1,1) bifurcation lesion are shown before stent placement; a Tryton stent will be placed from the proximal main vessel (P) into the side branch (S). Thin white lines demarcate the region of interest, whereas the red cross marks the common image point facilitating the 3-D reconstruction (A3); the most severe stenosis is located in the side branch ostium. B1-B3: Respective images post-procedure. Stenosis around the bifurcation is reduced, whereas minor obstruction is now located distal to the drug-eluting stent in the distal main vessel (M); PCI resulted in a slight increase in the 3-D distal bifurcation angle.
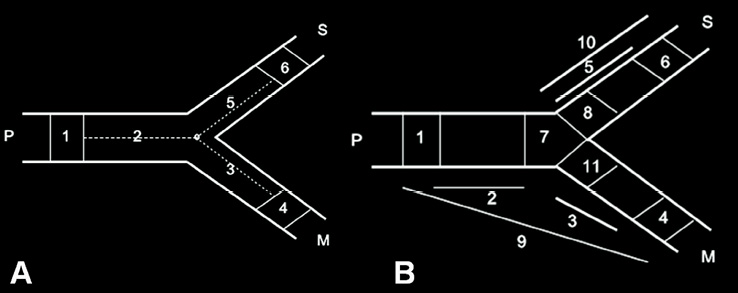
Figure 3. Bifurcation segment models in the Cardiovascular Angiography Analysis System (CAAS). Segments 2, 3 and 5 in both models reflect the segments where the stents were placed in the proximal main vessel (P), distal main vessel (M) and side branch (S), respectively; segments 1, 4 and 6 are corresponding 5 mm-long peri-stent segments. Segments 8 and 11 in the extended model (B) reflect 3 mm-long ostial segments for the side branch and distal main vessel, respectively.
In the cases where at least two angiographic images of the LM bifurcation separated by a viewing angle of ≥30° were available for both pre- and post-procedure, a 3-D reconstruction was also performed with a dedicated bifurcation algorithm10. Specifically, 2-D analysis was performed first using the 2-D angiographic image showing the best (optimal) view of the LM bifurcation. After saving the 2-D analysis results (numerical values and graphs) the vessel contours of this optimal view were imported into the 3-D analysis algorithm together with the second 2-D image, which still required contouring following the procedure already described. In the 3-D reconstructed image, segmentation of the region of interest and reporting of angiographic quantitative parameters were analogous with the 2-D analysis; thereby results from 2-D and 3-D analyses were directly comparable.
The depth of implantation of the Tryton stent was measured by QCA using the two middle markers as illustrated in Figure 4. The angiography film showing the positioning of the stent prior to implantation was analysed for all but 11 cases in which this was not recorded. The distances between the proximal middle marker and the carina and the distal middle marker and the carina were measured. A difference of ≥1 mm between the two measurements was identified as inappropriate implantation.
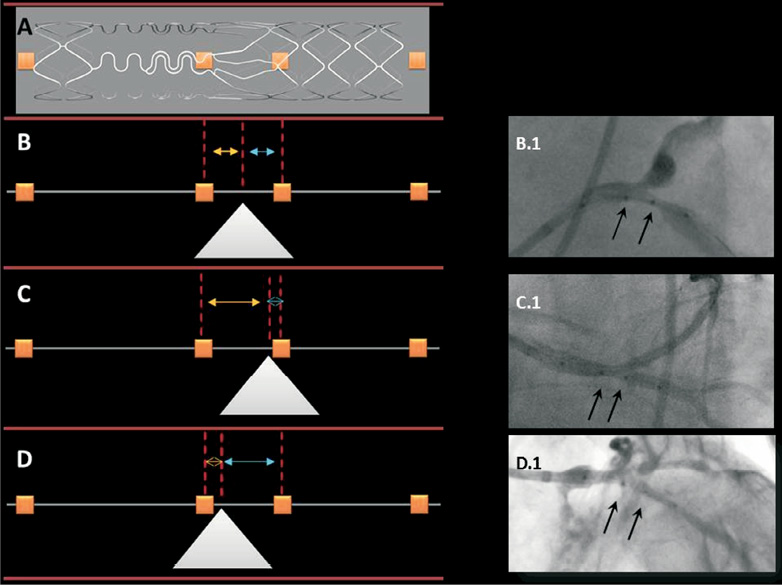
Figure 4. Depth of implantation assessement. The Tryton stent delivery system has four markers (depicted in orange boxes): the outer markers delineate the proximal and distal ends of the stent, while the two middle markers delineate the transition zone (A). The two middle markers (black arrows on angiographic images) should straddle the carina (white triangle) during implantation. Equal distances between the proximal middle marker (yellow arrow) and the distal middle marker (blue arrow) to a line perpendicular to the carina indicate appropriate implantation depth as exemplified in B and B.1. The implantation in C and C.1 is not deep enough (yellow>blue arrow), while that in D and D.1 is too deep (yellow
SYNTAX SCORE
SYNTAX score was calculated by an experienced analyst as previously described using the programme on the website www.syntaxscore.com11.
STATISTICAL ANALYSIS
Continuous variables are expressed as mean±SD or as median (interquartile ranges) whereas dichotomous data are summarised as counts and/or percentages. The paired t-test was employed for comparisons of quantitative parameters pre- and post-procedure and between 2-D and 3-D estimates of the same parameters. Statistical analysis was performed using SPSS software version 20 (SPSS, Chicago, IL, USA).
Results
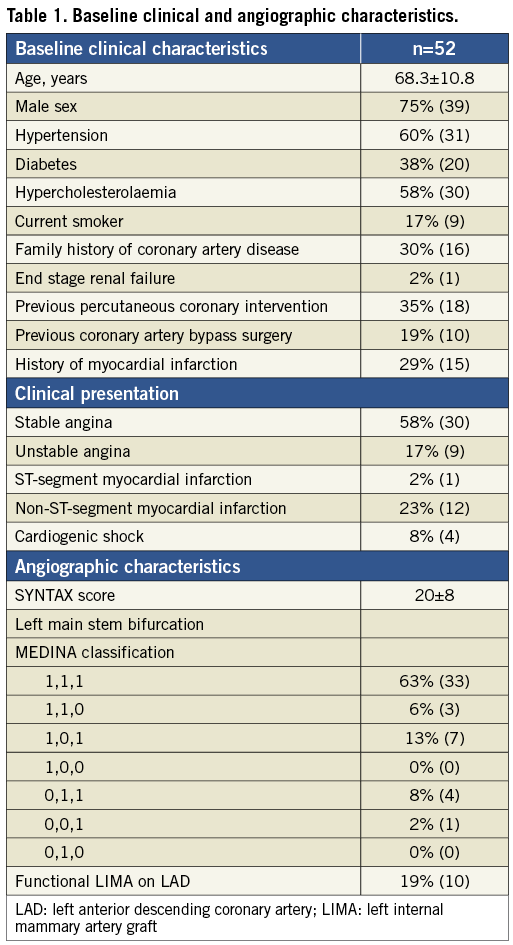
Fifty-two LM bifurcation lesions were included in the TRYTON LM registry between May 2008 and October 2011. Table 1 shows the baseline characteristics of the study cohort. The mean age of patients was 68.3±10.8 years and 75% were male. Fifty-eight percent of patients presented for PCI with stable angina while 25% presented with a myocardial infarction and 8% were in cardiogenic shock.
Coronary lesion characteristics are summarised in Table 1. The average SYNTAX score was 20±8 and 19% of patients had a previous CABG. Five patients had two bifurcation lesions that needed revascularisation. Eighty-five percent of lesions were true bifurcation lesions (1,0,1 or 1,1,1 or 0,1,1) .
The mean reference diameters for the LM stem, DMB and SB were 4.00, 2.63 and 2.48 mm, respectively. The mean DS was 39.5%, 36.2% and 41.8% for LM, DMB and SB, respectively. The mean angle between the LM and the SB was 139.4˚, while that between the DMB and the SB was 78.5˚.
All stents implanted during the procedure except for the Tryton stent were drug-eluting. Failure of delivery of the Tryton stent occurred in one patient despite repeated predilatation of a heavily calcified LM and a very tight lesion of the left circumflex (LCx) coronary artery. Eventually, a conventional drug-eluting stent (DES) was placed with a good angiographic result. For the rest of the cohort, a Tryton stent on a stepped balloon with a diameter of 3.5-2.5 mm was successfully deployed in 41/51 (80%) cases. In 11/51 (22%) cases, the distal zone of the Tryton stent was implanted in the left anterior descending (LAD) coronary artery. Six (55%) of these cases were protected by a left internal mammary artery. For the remaining five (45%), the LCx was larger in diameter than the LAD and the operator chose the latter as the “side branch”. The mean MV stent diameter was 3.4±0.4 mm with an everolimus-eluting stent employed in 30/51 (59%) of cases. FKB dilatation was performed in 48/51 (94%) bifurcation lesions. Table 2 summarises procedural parameters.
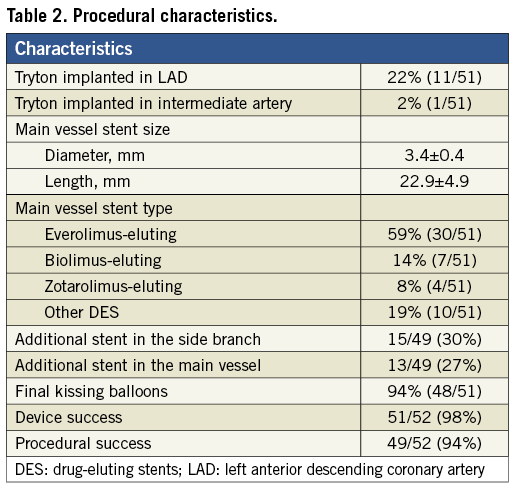
There were no device-related complications except for one delivery failure. Thus, the device success rate was 98%. Angiographic success was achieved in all patients (100%). One patient had a non-flow-limiting dissection in the LAD following deployment of a MV conventional stent that was used in combination with the Tryton stent for treatment of a LAD/diagonal branch bifurcation lesion. The dissection at the proximal edge of the stent was covered by placement of an additional stent and the operator went on to treat the LM bifurcation with a second Tryton stent, achieving a good result. The patient had no evidence of periprocedural infarction. A second patient also had two Tryton stents deployed to treat two bifurcation lesions during the same procedure with a good result. In total, three patients developed periprocedural MI without angiographic complications so that procedural success reached 94%.
QCA RESULTS
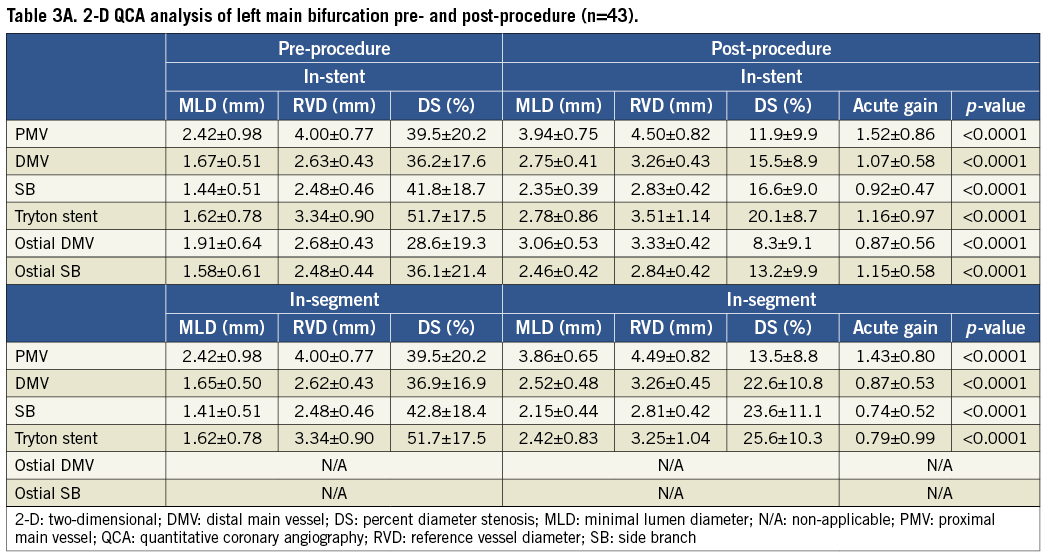
Two-dimensional analysis was performed in 46 cases. The other cases had to be excluded due to inadequate cineangiography either pre- or post-procedure (no clear view of the bifurcation, overlapping vessel segments, presence of angiographic guidewires). Results for diameter-derived parameters for the overall 2-D analysis are shown in Table 3A. Acute gain in the LM and in the SB was 1.52±0.86 mm and 0.92±0.47 mm, respectively.
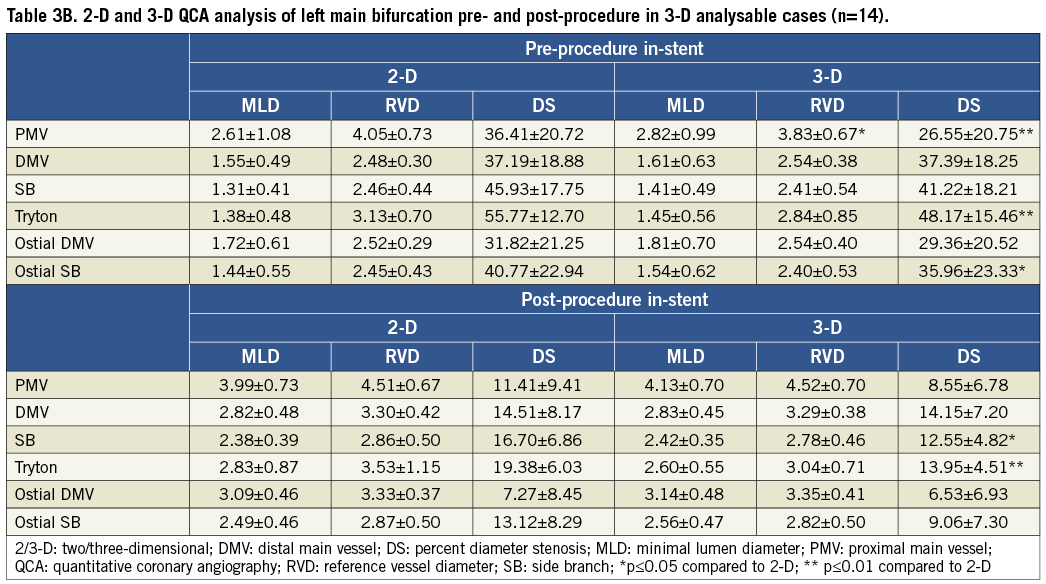
Three-dimensional analysis was feasible in 14 cases and results for diameter-derived parameters are shown in Table 3B. On average, two-dimensional BA values were larger as compared to the 3-D ones, both pre- and post-procedure (Table 4). However, the difference was significant for pre-PCI proximal BA values (145.1° vs. 132.4°, p=0.03) only.
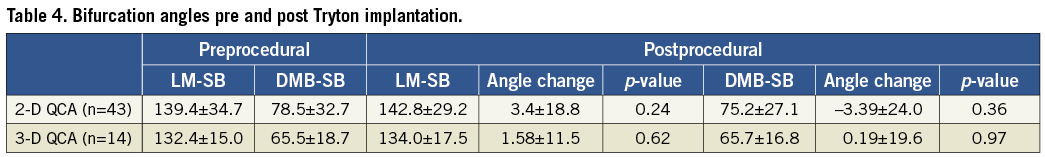
From the 46 angiography films analysed with 2-D QCA, the proximal BA changed by ≥5˚ in 65% (28/43) of cases, while the distal BA changed by more than ≥5˚ in 74% (32/43) of cases. For the distal BA, most cases with a change showed a narrowing of the angle and this occurred in 54% of the QCA cohort. On the other hand, the proximal BA most commonly widened (40% of cases) or remained the same (35%).
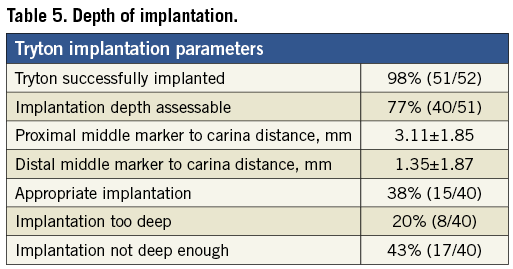
Depth of implantation of the Tryton stent was assessable in 77% (40/52) of cases. Depth was deemed appropriate in 38% (15/40). In 43% (17/40) of those assessed, the implantation was not deep enough, while in 20% (8/40) implantation was too deep. These results are summarised in Table 5.
CLINICAL FOLLOW-UP
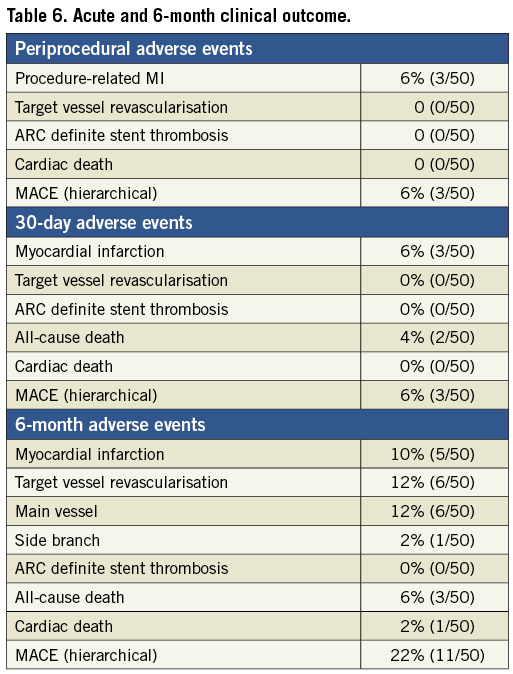
At a median of six months, one patient of the entire cohort treated with the Tryton stent was lost to follow-up. The events in the rest as well as the hierarchical MACE are reported in Table 6. One patient died of cardiovascular complications at 83 days after the index procedure. Two other patients died of non-cardiac causes, one from septic shock and the other from a gastrointestinal bleed. Apart from the three periprocedural MIs, two other patients suffered a NSTEMI at 55 days and 92 days post procedure, respectively. In the first patient, angiography revealed no obvious restenosis or culprit lesion, whereas in the second restenosis with a hazy lesion in the SB ostium was treated with balloon angioplasty. In total, six patients had TVR, all involving the SB, with one of them being referred for CABG because of concomitant MV and SB restenosis. All cases of TVR occurred in the side branch with QCA-derived reference diameter of <2.3 mm. The depth of implantation in cases of TVR was appropriate in two, too deep in two and not deep enough in two.
On further analysis of the baseline and follow-up angiography films of cases with restenosis, the following observations were made: all cases of restenosis involved the SB ostium. Evaluation of procedural steps revealed prolonged attempts at rewiring of the SB for FKB.
No cases of definite stent thrombosis were reported.
Discussion
Use of the Tryton side branch stent for treatment of LM bifurcation lesions was associated with a high rate of device and procedural success without complications in such a high-risk patient and lesion cohort. The angiographic result is excellent with optimal acute luminal gain in all three segments of the bifurcation including the distal MB and SB ostia as measured by 2-D and 3-D QCA. Although BA changes were observed to similar extents as previously described for two-stent techniques, they were not associated with adverse events at follow-up. At six months, TLR (12%) was due almost exclusively to SB ostium restenosis, an event that warrants in-depth evaluation.
Previous studies have shown that PCI of the LM has two distinct anatomical subcategories distinguished by their differential outcome in terms of restenosis. In fact, while treatment of disease limited to the shaft compares well with CABG results, that of the bifurcation is limited by excessive revascularisation rates. A LM substudy from the j-Cypher Registry found a three-year TVR rate of 3.6% for ostial lesions and significantly higher rates (17.1%) after bifurcation stenting with early-generation sirolimus-eluting stents12. Two-stent strategies including crush, culotte and T stenting provide better angiographic results at the expense of a more complex procedure with higher risk of complications and lack of superiority or even inferiority in terms of efficacy when compared to provisional T stenting. Reports of a 30.9% TVR rate at three years with two-stent techniques compared with a more acceptable 11.1% for provisional stenting are a matter of some concern12. An Italian survey also reported similar rates at two years with a similar difference between simple and more complex techniques (TLR-free survival 87% vs. 73%)13. Such trends are maintained up to five-year follow-up14. It is worth noting that the majority of two-stent techniques employed in these studies were crush and T stenting with under-representation of culotte stenting. The latter has the potential advantage of a better scaffolding of the distal vessel ostia and at the same time may minimise the number of unopposed struts at the bifurcation. In true bifurcation lesions with significant SB involvement, these features may be particularly advantageous. The Tryton stent facilitates the reverse culotte technique and, as shown in previous reports, it simplifies the interventional technique leading to a low intraprocedural complication rate and excellent angiographic results. In our current registry, these theoretical advantages still resulted in a TLR rate of 12% at six months. Some important device-dependent and operator-dependent features have to be considered in order to understand how these may interact to affect clinical outcome.
DEPTH OF IMPLANTATION
The middle markers on the Tryton stent delivery system are essential to guide the desired depth of implantation of the device. Straddling the carina between the two markers allows correct positioning of the stent transition zone, resulting in good scaffolding of the SB ostium. This zone has a lower radial strength than the SB zone due to the design, which on the other hand allows for easier recross of wires, balloons and stents across it and along the MV. Therefore, a balance has to be sought, as implanting the device too deep will result in poor SB ostial scaffolding while too shallow an implantation will result in difficult MV rewiring. Most operators in our series preferred to keep the major part of the transition zone in the MV. This strategy probably provides better scaffolding of the SB ostium, while recrossing into the relatively large MV was not associated with any significant difficulty. Theoretically, this issue would be best explored with the use of intraprocedural intravascular ultrasound (IVUS) or optical coherence tomography (OCT). However, it should be noted that inappropriate implantation depth was not predictive of restenosis up to six months post procedure.
STENT SIZING
Small branch vessels treated with the Tryton stent were more likely to have restenoses at follow-up. Indeed, five of the seven cases with restenosis at six months occurred in vessels less than 2 mm by QCA. Introduction of a drug-eluting version of the Tryton stent may potentially overcome this problem in vessels ≤2.5 mm in diameter.
CHANGE IN BIFURCATION ANGLE AFTER TRYTON-CONVENTIONAL STENT IMPLANTATION
Cardiac motion may change BA, namely widening the angle between the LM and the LCx, and simultaneously narrowing the angle between the LAD and the LCx by about 8˚15. PCI of the LM bifurcation also changes the angle in most instances, narrowing it by about 5˚. Our study showed similar changes with the use of a dedicated bifurcation stent.
In a recent 3-D QCA study, a change of ≥5° between either the PMB and SB or DMB and SB after stenting was considered significant. Of the 102 bifurcations included in the study, 15 involved the LM. The angle between the DMB and SB changed significantly. Of note, the angle became wider with the use of complex as compared to simple bifurcation stenting techniques. However, this was not associated with adverse events. On the other hand, widening of the angle between the PMB and SB was associated with fewer events, while angle narrowing resulted in a higher rate of complications16. In our study, neither the angle at baseline nor a change in the angle after PCI was associated with adverse events, including restenosis. This, however, does not exclude a higher postulated risk of bifurcation restenosis with very wide angles between the DMB and SB, as scaffolding at the point of bifurcation may be reduced. Such bifurcations are under-represented in our study cohort, reflecting appropriate selection of cases based on the anatomy by the treating interventional cardiologist. Therefore, the impact of bifurcation angle change on outcome in LM intervention in appropriately selected cases certainly remains limited.
OTHER PROCEDURAL CHARACTERISTICS THAT MAY HAVE AN IMPACT ON OUTCOME
Technical strategies to ensure long-term patency include lesion preparation with rotational atherectomy if lesions are significantly calcified, balloon predilatation17, use of cutting balloons when needed, implantation of stents at relatively high pressures, and post-dilatation with non-compliant balloons. Use of IVUS imaging18 may be of help in assessing the need for predilatation or atherectomy, in appropriately sizing the vessel, and to evaluate the need for further post-dilatation after stenting in order to ensure good acute luminal gain. In patients undergoing bifurcation lesion PCI, FKB dilation has a positive effect on acute angiographic results, which seems to translate into better clinical outcome19. Indeed, the Nordic-Baltic Bifurcation Study III showed that FKB dilation reduced angiographic SB restenosis at six months in bifurcation lesions other than distal LM, especially in patients with true bifurcations (7.9% vs. 15.4% [p=0.039] for all bifurcations and 7.6% vs. 20.0% [p=0.024] for true bifurcations)20. Prolonged attempts at rewiring the SB for FKB were noted on detailed analysis of the Tryton cases needing revascularisation at follow-up in the current registry. We can speculate that this may be the result of passage of the wire behind the struts of the SB ostial portion of the Tryton stent (presumably at the transition zone). If that is the case, kissing inflations would crush the transition zone against the ostium, resulting in reduced carina scaffolding despite the optimal acute angiographic result and restenosis. Another possible cause of SB restenosis could be the lack of an antiproliferative drug coating on the Tryton stent implanted in a site at high risk of restenosis, such as the LCx ostium.
QCA FOR BIFURCATION ANALYSIS
The mean values for diameter stenosis established by QCA analysis in the cohort are less than 50% in all three segments of the bifurcation. This implies that such measurements were not used in isolation to determine whether the LM bifurcation should be treated or not. Discrepancy between eyeballing and QCA (overestimation of eyeballing), use of complimentary parameters such as lesion instability, pressure drop on engagement of the LM and other physiological parameters may have instigated treatment. Angiographic derivation of the reference vessel diameter prior to intervention may underestimate the true lumen diameter, especially in diffusely diseased segments. In fact after stent implantation we observed an enlargement of the RVD. This occurs commonly with the LM since it is an end vessel and involvement starting from the ostium precludes measurement of the true RVD by angiography on the pretreatment film. Despite these plausible reasons for the low DS in treated LM bifurcations, we still cannot exclude overtreatment, i.e., intervention of apparently non-significant lesions with the interventionalist being keen to treat if there is doubt about the significance as it is the LM.
LIMITATIONS
The present study has the intrinsic limitations of a registry. These cases are selected and therefore may not reflect use in all-comer LM lesions. In particular, the limited Tryton stent sizes available at the time of implantation may have affected the outcome. Therefore, it is more likely that the present results reflect stent performance in the range of vessel sizes treated in our cohort. There was no control group to compare the use of this dedicated bifurcation stent and stenting with other devices and techniques. Moreover, the number of patients enrolled in the registry is too limited to draw conclusions on definite and reproducible clinical outcome rates. Also, the majority of patients enrolled had no angiographic or other invasive imaging follow-up. QCA was not possible in 100% of the cohort for the reasons explained above. The use of intravascular imaging techniques (IVUS or OCT) was not available in the majority of cases and would have been useful for better evaluation of the cases of stent failure.
Conclusions
Use of the Tryton stent for treatment of LM bifurcation disease in combination with a conventional drug-eluting stent is feasible and results in optimal acute angiographic results. Safety of the procedure and six-month clinical outcome are acceptable in this high-risk lesion PCI; further prospective safety and efficacy studies with intravascular imaging as well as long-term outcomes of this strategy are warranted.
Conflict of interest statement
The authors have no conflicts of interest to declare.

