Abstract
Aims: Catalytic iron is associated with high oxidative stress during vascular injury. We measured catalytic iron in patients with suspected acute coronary syndromes (ACS) and healthy volunteers to evaluate its utility in early detection of patients with acute myocardial infarction (MI) and predicting major adverse cardiac events (MACE).
Methods and results: Catalytic iron was measured on admission and 24 hours later in 127 patients with acute MI, 51 patients with suspected ACS without MI, and 250 healthy volunteers. Descriptive and decision statistics were performed for catalytic iron and troponin I.Catalytic iron levels at presentation were 1.5+2.0 µmol/l, 0.2+0.16 µmol/l, and 0.1+0.06 µmol/l for acute MI, suspected ACS without MI, and normals, respectively p<0.0001. Catalytic iron was elevated in all patients with MI at presentation. At a cutpoint of 0.30 µmol/L, the sensitivity, specificity, and diagnostic accuracy for identifying MI was 84%, 95%, and 92%, respectively. Increase in catalytic iron at 24 hours compared to baseline was associated with MACE at 30 days.
Conclusions: Catalytic iron identified all patients with acute MI at presentation and serial elevation was independently associated with MACE. This biomarker of vascular injury is useful in the rapid serologic assessment of patients with suspected ACS.
Introduction
Despite advancements in the clinical evaluation of suspected acute coronary syndromes (ACS), there remains a need for rapid diagnosis to facilitate urgent treatment. The emergency assessment based on patient history, electrocardiogram (ECG), and blood biomarkers is the cornerstone in the identification of high-risk subsets who would potentially benefit from therapeutic interventions including antiplatelet1 and antithrombotic agents2 and early reperfusion and revascularisation strategies3,4. Cardiac troponin I and T are the most widely used biomarkers of myonecrosis and have an established pivotal role in identifying patients with high-risk ACS5,6. Patients with ST elevation myocardial infarction (MI) are readily identified by the ECG, and hence receive benefits of early reperfusion strategies. Non-ST elevation MI patients also benefit from early invasive strategies but the intervention is usually delayed in part because of the absence of a reliable early biomarker in the setting of a non-diagnostic ECG7,8.
Plaque rupture and atherothrombosis triggers ischaemic myocardial injury with subsequent myonecrosis and a biomarker of this vascular damage has been the subject of intense research. A spike in systemic oxidative stress is an important trigger for such a vascular insult and catalytic or labile iron is a potent catalyst. The catalytic iron pool consists of chemical forms that can participate in redox cycling and has been associated with oxidative stress9-11. Critical to iron’s importance in biological processes is its ability to cycle reversibly between its stable ferrous and reactive ferric oxidation states. This precise property, which is essential for its functions, also makes iron potentially hazardous in that it enables it to participate in the generation of powerful oxidant species such as hydroxyl radical and/or reactive iron-oxygen complexes such as ferryl or perferryl ion9. Catalytic iron has been implicated in several disease states including acute and chronic kidney disease12-14, myocardial reperfusion injury15,16, and neurodegenerative disorders17. Since catalytic iron has been implicated causatively with vascular disease processes, one may anticipate that a change in catalytic iron may precede the onset of injury. We evaluated the ability of serum catalytic iron in patients with suspected ACS to identify the subset of patients with acute MI at the time of their presentation and to identify those who may have a complicated course over the next 30 days.
Methods
Study population
Between April 2006 and October 2007, 127 patients with acute myocardial infarction, 51 patients with suspected ACS without MI and 250 healthy volunteers were enrolled at Bhailal Amin General Hospital, Baroda, India. Each patient’s detailed clinical history and physical examination was recorded. An initial ECG was obtained and assessed for ST segment shifts and ischaemic changes. Blood sampling for cardiac biomarkers including troponin I and serum catalytic iron was conducted at the time of presentation and 24 hours after admission. Both subjects and investigators were blinded to the catalytic iron results. Myocardial infarction was defined according to standard criteria18. All patients then underwent standard cardiac care depending on the clinical condition at the time of presentation according to the discretion of the treating physician.
Group A consisted of 127 patients who were diagnosed with acute MI based on history, ECG, and serial elevation of troponin I characteristic of myocardial injury. Group B consisted of 51 patients with chest pain who were suspected to have ACS but had normal troponin I at presentation and at 24 hours. We measured catalytic iron in a contemporary cohort of 250 healthy volunteers (Group C) who took part in a health survey of government employees in the Nadiad Region of India during the same time frame in which we obtained the samples from Groups A and B. Groups A and B were followed for at least 30 days and adverse events including death, reinfarction, and stent thrombosis were adjudicated by two independent cardiologists after chart review blinded to serum catalytic iron and research troponin I assay results. This study received permission from the Bhailal Amin General hospital’s ethics and review board and informed consent was obtained in all cases.
Blood sampling
At the time of enrolment and at 24 hours after admission, 10 ml of blood was collected by venipuncture in a plain non-barrier tube and centrifuged at 1400-2000 RCF for 10 min at 4°C. The serum component was frozen and transported on dry ice to Muljibhai Patel Urological Hospital, Nadiad, Gujarat, India where the samples were stored at –70°C. All of the samples were then thawed at the time of analysis.
Biochemical analysis
Troponin I was measured using a immunoenzymometric assay using a kit provided by Calbiotech, Inc., Spring Valley, CA, USA (P.N. TI015CM). Catalytic iron was measured using the bleomycin-detectable assay based on the observation that the antitumor antibiotic bleomycin, in the presence of iron and a suitable reducing agent, binds to and degrades DNA with the formation of a product that reacts with thiobarbituric acid to form a chromogen. The assay therefore detects iron complexes that are capable of catalysing free-radical reactions in biological samples19. The reactions were carried out in one-half the recommended volume and performed in disposable polypropylene tubes to avoid iron contamination from external sources. All of the reagent solutions except the bleomycin were treated overnight with chelex (Bio-Rad Laboratories, Inc., Hercules, CA, USA) (300 mg for 10 ml solution) to remove any iron in the chemical reagents.
Statistical analysis
Patients were studied in three groups as described above. With the sample size limited by the number of cases of acute MI (n=127), our study had 97% observed power (alpha=0.01, two-tailed) (Sample Power, Version 1.0) for the differences between Groups A and B. Comparisons of catalytic iron and troponin I values in various groups were performed by t-test for independent samples and ANOVA with Bonferroni correction was used for analysis of variance. We log-transformed values for catalytic iron and troponin to reduce effects of skewness in the distribution and used them for statistical testing but reported the untransformed values in text, tables, and figures. To evaluate the role of catalytic iron in the diagnosis of acute MI, decision statistics were calculated (sensitivity, specificity, positive and negative predict value, accuracy) after construction of a receiver-operating-characteristic (ROC) curve and identification of an optimal cut point. Patients in groups B and C who did not have acute MI were considered as controls. Multiple logistic regression was used to evaluate the independent association between catalytic iron elevation over the first 24 hours and adverse cardiac events at 30 days while controlling for baseline confounders. The statistical analysis was performed using the software SPSS version 15 (SPSS Inc., Chicago, IL, USA).
Results
The baseline characteristics and 30 day outcomes of the three groups are shown in Table 1.
Among the 127 patients in group A with acute MI, 61 (48.0%), 43 (33.9%), 23 (18.1%) presented ≤ 3, 4-12, and > 12 hours after the onset of chest pain, respectively. Of the 127 patients in Group A, 63 (49.6%) patients at the time of presentation had ECG evidence of ST elevation MI, 64 (50.4%) patients had non-ST elevation MI, and all had characteristic elevations of troponin I over the course of hospitalisation. A total of 13 (10%) and 95 (74%) of the acute MI patients were treated with thrombolysis and underwent percutaneous coronary intervention, respectively. Eighteen (14%) patients were detected to have multivessel coronary artery disease and underwent coronary artery bypass surgery. The 51 patients in Group B with normal serial troponin I estimations underwent non-invasive testing with a treadmill test in eight (34%) and dobutamine stress echocardiography in 33 (66%). One patient in this group underwent a coronary angioplasty two weeks after the index admission following recurrent chest pain.
Box plots of the baseline catalytic iron measurements are shown in Figure 1.
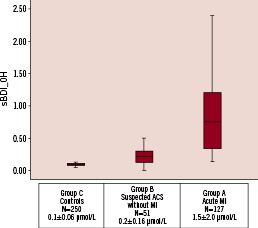
Figure 1. Box plots showing median levels of catalytic iron measured at baseline in the emergency department in the three groups of patients. Boxes show interquartile ranges and the bar represents highest and lowest values.
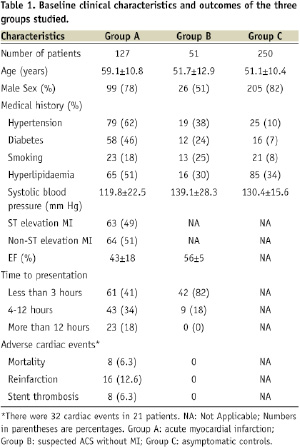
Group C, healthy volunteers, had a mean (±standard error of the mean) catalytic iron level of 0.1+0.06 µmol/L which is similar to the previously published data in the normal population. Patients in group B with suspected ACS without MI had catalytic iron levels of 0.26±0.16 µmol/L. Patients in group A with acute MI had a mean baseline catalytic iron level of 1.55±2.02 µmol/L. There was a highly statistically significant difference between patients with acute MI, ACS without MI, and healthy volunteers (P<0.0001 for group A versus B and A versus C).
Patients with acute MI who presented ≤ 3 hours (Figure 2) after the onset of chest pain had a mean baseline catalytic iron level of 1.82±1.71 µmol/L.
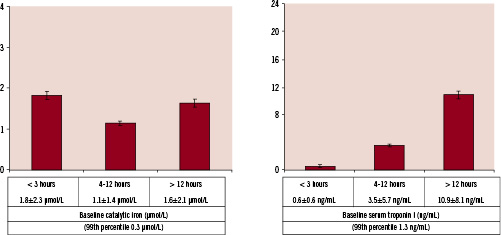
Figure 2. Catalytic iron and troponin I measured at baseline at the time of presentation in the three groups divided by the time of symptom onset to presentation in emergency department.
This level was significantly higher than both controls and those with suspected ACS without MI. The baseline troponin I level among this subset of patients was 0.6±0.6 ng/ml (99th percentile 1.3 ng/ml). Serial troponin I estimations in this subset subsequently rose when assessed 24 hours after presentation (7.1±1.1 ng/ml), confirming that these patients had acute MI with initially normal troponin values.
The ability of catalytic iron measurement to identify acute MI was tested using ROC analysis (Figure 3) and the optimum cutpoint was determined to be 0.3 µmol/L.
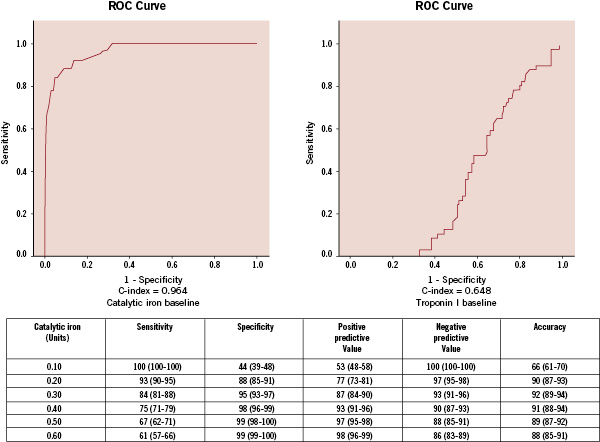
Figure 3. Receiver-operating-characteristic curve for various cut-off levels of catalytic iron in differentiating between acute MI and ACS without MI.
At this level, the sensitivity was 84%, specificity 95%, positive predictive value 87%, negative predictive value 93%, and diagnostic accuracy 92% for the diagnosis of acute MI.
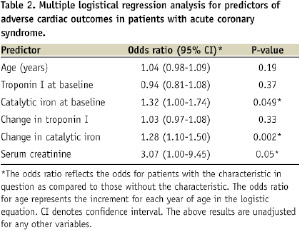
Patients with adverse cardiac outcomes (defined as death, reinfarction, stent thrombosis) had significant increases in levels of catalytic iron from baseline to 24 hours after presentation compared to those without (mortality: 6.5±2.6 versus 0.3±0.2 µmol/L, P<0.0001; reinfarction: 3.5±1.7 versus 0.1±0.2 µmol/L, P<0.0001; stent thrombosis: 5.0±2.8 versus 0.3±0.2 µmol/L, P<0.0001). Multivariate analysis found that an elevation of catalytic iron at 24 hours from baseline was strongly associated with adverse cardiac outcomes (OR=1.28, 95% CI 1.10-1.5, P<0.002) along with serum creatinine and serum catalytic iron at baseline when controlling for baseline and change in troponin I (Table 2).
Discussion
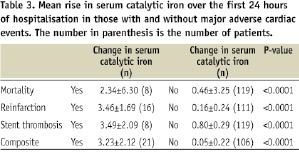
We found that baseline catalytic iron had favourable decision statistics for acute MI particularly in those patients presenting ≤ 3 hours after the onset of chest discomfort before the characteristic elevation in troponin I. In addition, an elevation of catalytic iron over the next 24 hours was independently associated with an increased risk of adverse events (death, reinfarction, stent thrombosis) at 30 days (Table 3).
Patients with suspected ACS who developed acute MI identified by this novel marker of oxidative injury are a population which has been demonstrated to benefit from early initiation of aggressive antithrombotic, antiplatelet, and reperfusion therapies.
The first account of the use of a biochemical marker in the study of myocardial injury was published by La Due and colleagues in the journal Science in 195420. Over the past 54 years, many attempts have been made to identify patients with acute MI early beyond conventional biomarkers in order to offer specific benefits from earlier administration of antiplatelet, antithrombotic and revascularisation therapies1-4,20. In this first study of catalytic iron in patients suspected ACS, this novel biomarker appears to show promise particularly in patients with chest pain and nondiagnostic ECGs where the initial diagnosis may be a dilemma.
Recently various biomarkers in addition to cardiac troponins have been used to predict adverse cardiac outcomes in patients presenting with ACS21. Most notably, B-type natriuretic peptide measured at baseline in ACS when elevated over 80 pg/ml has been associated with higher in-hospital mortality and the later development of heart failure but has not been associated with reinfarction or stent thrombosis22,23. In contrast, we found the rise catalytic iron over 24 hours was predictive of ischaemia adverse outcomes and was independent of anticipated determinants of mortality including age and the troponin I values.
The possibility that iron plays a role in cardiovascular disease was postulated by J.L. Sullivan in 198124. It has been shown that the postsecretory, iron-dependent oxidative modifications of lipoproteins constitute a crucial step in atherogenesis25. Our findings may suggest that spikes in catalytic iron and systemic oxidative stress coincide with atherosclerotic plaque rupture, coronary vasospasm and vascular damage resulting in ACS; appearing initially to be the cause rather than the effect of myocardial injury26,27. An initial elevation in catalytic iron may trigger apoptosis of endothelial cells and thus participate early in the plaque rupture process28. Although events culminating in apoptosis commonly involve biologically active organic molecules such as ligands and auto-destructive proteins, inorganic signals relevant to physiological stress such as catalytic iron and perhaps other trace metals could trigger apoptosis. Changes in the serum concentration of other trace metals have been associated with acute MI. For example, elevated serum copper levels have been correlated with the extent of myocardial damage29. Conversely, a reduction in serum zinc levels following MI has been associated with adverse outcomes30. We hypothesise that initiation of vascular damage itself may cause further release of catalytic iron, and perhaps other trace metals, which participate in reactions that generate free radicals that mediate oxidative damage to the myocardium31 thus expanding the zone of tissue death.
Our study has all of the limitations of small observational studies. The patients in Group B with suspected ACS without MI did not undergo coronary angiography and hence it did not allow us to distinguish between unstable angina and true non-cardiac chest pain. The intermediate elevation of catalytic iron in group B reflects this heterogeneity. In addition, we did not inquire about outpatient consumption of supplemental iron or assess for iron re-utilisation or storage disorders. Since catalytic iron is released from cells into a small measurable blood pool after oxidative injury, we do not believe our results are confounded by dietary, bone marrow, or hepatic sources of iron which circulate in bound forms. Lastly, we did not assess infarct size, which may be an important determinant of the degree of myocardial oxidative injury and thus be the core problem reflected by the degree of catalytic iron elevation.
The present study suggests potential clinical benefits of using catalytic iron as an early biomarker of plaque rupture and vascular damage. Use of this biomarker at the time of presentation of chest pain may accurately identify patients with acute MI, thus helping institute early use of beneficial therapeutic strategies. Future studies will need to validate and characterise the serial elevation of catalytic iron and its relationship to infarct size and its association to in-hospital and 30-day adverse events.
Acknowledgements
The concept of the study was developed by Drs. Mohan Rajapurkar, Suhas Lele and Sudhir Shah. Critical review, further manuscript development, and additional analyses were directed by Dr. McCullough. The laboratory analysis was undertaken at the clinical laboratory of Muljibhai Patel Urological Hospital, Nadiad, India, and were performed by Dr. Banibrata Mukhopadhyay. The study was funded by the Muljibhai Patel Society for Research in Nephro-Urology. The data in paper forms and in computer has been stored with the Muljibhai Patel Society for Research in Nephro-Urology. The statistical analysis was done by Mr. Shashikant Chinchole, M.Sc. and Dr. Ashok Shanubhogue, M.Sc., Ph.D. Finally we acknowledge and thank Ms. Cindy Reid for assistance in preparing the final manuscript.

