Abstract
Aims: The present report describes a novel coronary fractional flow reserve (FFR) system which allows FFR assessment using a rapid exchange microcatheter (RXi).
Methods and results: The RXi microcatheter is compatible with standard 0.014” coronary guidewires facilitating lesion negotiation and FFR assessment in a wide range of coronary anatomies. In case of serial lesions, a microcatheter would have the important advantage of allowing multiple pullbacks while maintaining wire access to the vessel. The RXi is a fibre-optic sensor technology-based device. This technology might allow reduction in signal drift. The RXi microcatheter’s fibre-optic sensor is located 5 mm from the distal tip. The microcatheter profile at the sensor site is 0.027”×0.036”. The segment of the catheter which is intended to reside within the target lesion is proximal to the sensor and has dimensions decreased to 0.020”×0.025”; these dimensions are comparable to a 0.022” circular-shaped wire.
Conclusions: The RXi microcatheter FFR system represents a novel technology that could allow easier lesion negotiation, maintaining guidewire position, facilitating pullbacks for assessment of serial lesions and simplifying the obtainment of post-intervention FFR measurements. The optical sensing technology could additionally result in less signal drift. Further investigations are required to evaluate the clinical value of this technology fully.
Introduction
Fractional flow reserve (FFR) is an established functional index for evaluation of the severity of epicardial coronary stenosis and myocardial ischaemia1. The clinical benefit of FFR-guided PCI compared to angiography-guided PCI has been demonstrated in several investigations1,2.
Rapid exchange FFR system
The ACIST RXi™ Rapid Exchange FFR system with Navvus™ MicroCatheter (ACIST Medical Systems, Eden Prairie, MN, USA) incorporates fibre-optic-based sensor technology to assess FFR, at variance with standard pressure wire systems relying on piezoresistive (electrical) sensor technology.
The microcatheter is compatible with standard 0.014” guidewires, with a guidewire lumen diameter of 0.016” and a lesion entry profile of 0.022” (Figure 1, Figure 2, Table 1). The catheter’s fibre-optic sensor is located 5 mm from the distal tip and the radiopaque marker at 2.5 mm. The microcatheter is elliptically shaped at the sensor site with a profile of 0.027”×0.036” (Figure 2, Figure 3). The segment of the catheter that should reside within the lesion is proximal to the sensor, where the catheter dimensions decrease to 0.020”×0.025”. These dimensions are comparable to a 0.022” circular-shaped wire.
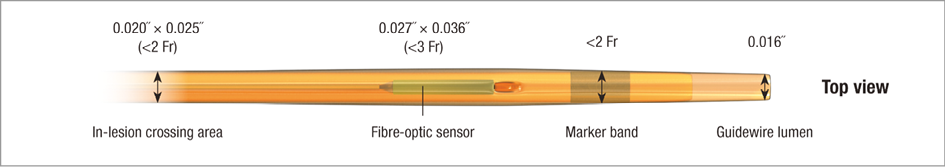
Figure 1. The Navvus Rapid Exchange FFR MicroCatheter. The microcatheter is compatible with standard 0.014” guidewires with a lumen diameter of 0.016”. A radiopaque marker band and the fibre-optic sensor are shown proximally.
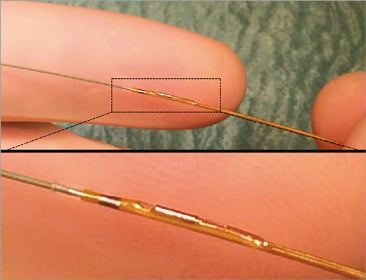
Figure 2. The Navvus Rapid Exchange system and profile. Upper panel: Navvus MicroCatheter system with a standard wire (BMW) showing the profile of the microcatheter. Lower panel: magnification of the tip of the microcatheter with the fibre-optic sensor.
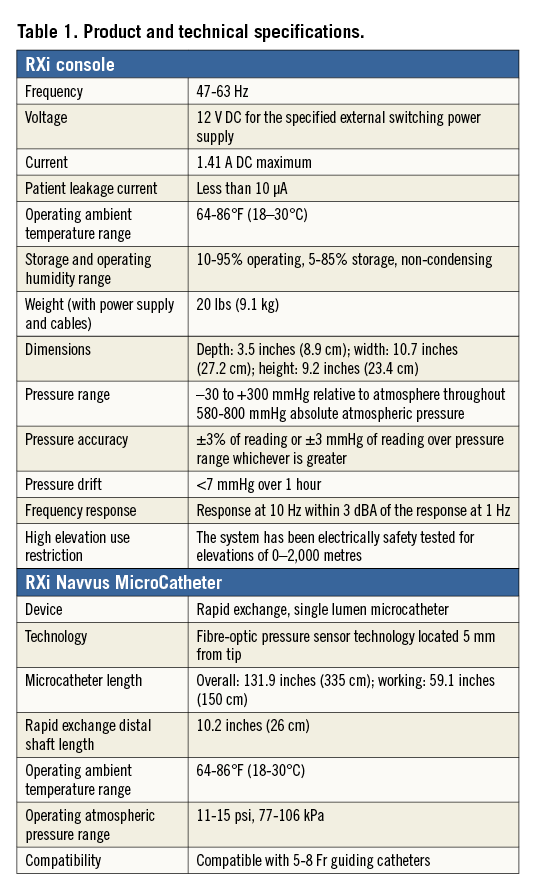
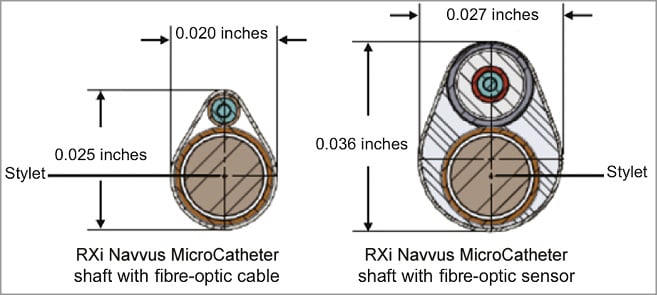
Figure 3. Profile and structure in cross-sectional view of the Navvus microcatheter at the level of the fibre-optic sensor and at the lesion site. At the level of the fibre-optic sensor the microcatheter has an elliptically shaped profile with dimensions of 0.027”×0.036”. At the lesion site the profile of the microcatheter decreases to 0.020”×0.025”; these dimensions could be considered comparable to those of a 0.022” circular-shaped wire.
Fibre-optic sensing technology
The RXi system fibre-optic sensor technology measures pressure by using light to assess the width of a cavity within the sensor. When blood pressure is applied to the sensor flexible distal face, the width of the cavity is reduced with increasing pressure. Pressure is sensed by measuring the cavity width using a Fabry-Pérot white-light interferometer3. A light source is guided into an optical fibre and then into a coupler (Figure 4A). Its output is linked to the optical fibre sensor (OFS). At the core of the OFS is the interferometer, which consists of two parallel, perfectly flat semi-reflecting mirrors separated by the cavity width, which varies with blood pressure. Light passing through the first mirror is reflected back and forth within the cavity multiple times (Figure 4B). However, at each reflection, a small fraction of the beam escapes the interferometer creating a large number of parallel beams of light (R1, R2, … Rn) (Figure 4B) coupled back into the optical fibre. The light returned to the system is therefore “encoded” with the width of the cavity, since each reflection is delayed by an integer multiple of cavity widths.
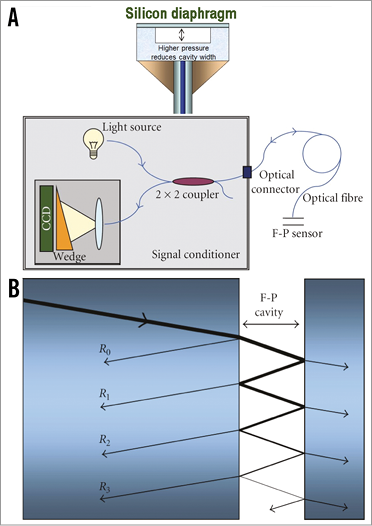
Figure 4. Fibre-optic pressure sensing technology. A) Fabry-Pérot white-light interferometer and coupler. B) A small fraction of the incident beam escapes the interferometer creating a large number of parallel beams of light. Reproduced with permission from Pinet3.
The returned light enters the signal conditioner, where light is directed by the coupler (Figure 4A) to an optical system where the light is spread over a wedge. Where the width of the wedge exactly equals the width of the cavity, constructive interference results since the delays in optical path length are recreated. At all other points destructive interference results in a reduced signal level. A charge-coupled device (CCD) records the resulting pattern. Thus, finding the cavity width corresponding to the blood pressure consists of looking into the position of the maximum peak in the interference pattern.
This technology is insensitive to changes in light transmission throughout the path, resulting in a reliable and consistent pressure waveform, which translates into pressure measurement unaffected by minor electrical changes that could cause signal drift as observed with current pressure wire technology.
Theoretical advantages and pitfalls of a microcatheter FFR system
Several theoretical advantages may be proposed for the present technology. 1) Coronary anatomies can be challenging because of excessive tortuosity, acute angulation and calcification, which may preclude navigation with dedicated FFR pressure wires. The ability to use more sophisticated hydrophilic-coated guidewires may facilitate lesion negotiation and allow FFR assessment in a wider range of anatomies. 2) Fractional flow reserve is a reliable functional assessment of the severity of coronary lesions. However, in diffusely diseased vessels the evaluation of each individual narrowing is affected by haemodynamic interaction among serial stenoses. Previously, equations were developed, and subsequently validated in vivo, to predict the FFR values of each stenosis when two serial lesions were present in a vessel4. More recently, FFR gradient across an individual stenosis during pressure-wire pullback was proposed as a surrogate of the relative functional severity of each stenosis in coronary tandem lesions5. This technique was observed to be associated with a reduction in unnecessary interventions6. However, this methodology requires pressure-wire pullback with inherent loss of wire access to the vessel and the need to re-cross the lesion with all the associated limitations and risks. A microcatheter compatible with any 0.014” wire would have the important advantage of allowing multiple pullbacks while maintaining wire access. 3) FFR measured after coronary stenting has been reported to be a strong independent predictor of clinical outcomes, providing a clinically relevant tool to evaluate the results of coronary interventions. However, measuring FFR after stenting either requires re-crossing the stent with the pressure wire (which introduces the inherent risk of the wire being placed behind the struts) or, if stenting was performed over the pressure wire, reconnecting the FFR system, which increases the likelihood of signal drift. The present microcatheter allows FFR measurement after stenting using the same 0.014” guidewire used during stent implantation, eliminating the need to re-cross the lesion or to disconnect the system, theoretically reducing the occurrence of signal drift. In addition, no re-calibration is currently recommended for serial microcatheter re-insertions in the coronary vessel.
On the other hand, a possible limitation of the microcatheter is the fact that, since the RXi Navvus has a higher profile than a 0.014” coronary guidewire, the device itself could mildly increase an existing stenosis and hamper blood flow compared to an FFR guidewire alone, especially in a severe stenotic lesion. However, FFR is indicated for assessment of intermediate lesions of unknown significance and not usually needed in case of subtotal atherosclerotic lesions. In coronary arteries with a reference luminal diameter ≥2.5 mm and an intermediate stenosis, a microcatheter with dimensions of 0.020”×0.025” is expected to have no significant haemodynamic effect.
Preliminary clinical data
We performed a preliminary evaluation of the RXi microcatheter system, comparing it to results obtained with a standard FFR pressure wire. A total of 15 patients with intermediate de novo atherosclerotic disease in native coronary arteries were sequentially evaluated with currently available pressure wire FFR systems and the RXi microcatheter FFR system.
Values showed a good positive correlation and good agreement between methodologies (Figure 5). No complications were observed.
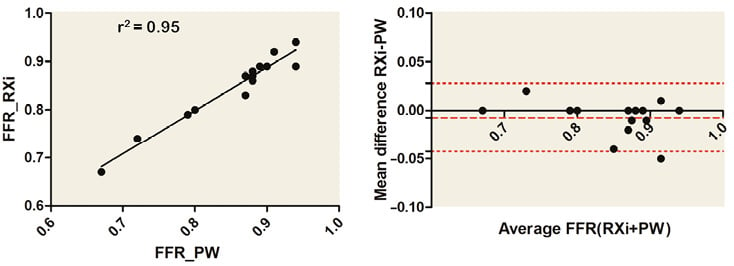
Figure 5. Correlation and agreement between standard pressure wire FFR system and the RXi microcatheter FFR system. Good positive correlation and agreement between measurements performed with either the RXi Navvus MicroCatheter system or a standard FFR pressure wire (PW).
Given the low number of patients, these data should be considered within the present report as additional information, descriptive and hypothesis-generating. The performance of the present device in coronary arteries with reference vessel diameters <2.5 mm should be clearly defined in further clinical studies.
Limitations
The present manuscript is intended to be a technical presentation of a novel technology. Therefore, quantitative measurements are purely descriptive and further in vitro and in vivo investigations are needed to elucidate fully the additional clinical value of such technology. The theoretical advantage of reduced signal drift should be extensively evaluated and quantified. In addition, comprehensive validation studies comparing standard FFR technique with this new system would be necessary before the large clinical evidence acquired for FFR pressure wires could be transferred to the microcatheter system. Additional clinical uses of the present technology might be considered in different settings comprising valves and peripheral vascular disease.
Conclusions
The RXi and Navvus MicroCatheter FFR system represents a novel technology to simplify fractional flow reserve assessment. The RXi system and Navvus microcatheter technology could facilitate FFR assessments because a workhorse wire can be used to access the lesion. This novel approach allows the wire to remain in place for subsequent assessment of serial lesions, the intervention itself, and post-intervention FFR. Additionally, the optical sensing technology could result in less signal drift. Further studies are required to evaluate fully the clinical value of this technology.
| Impact on daily practice The possible implications for clinical practice related to the use of the RXi microcatheter could comprise an expanded adoption of FFR-guided PCI, a more accurate assessment of the haemodynamic relevance of serial coronary lesions, and an easier post-PCI FFR assessment. |
Guest Editor
This paper was guest edited by Andreas Baumbach, MD; Department of Cardiology, Bristol Heart Institute, Bristol, United Kingdom.
Conflict of interest statement
The authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

