
Abstract
Aims: Patients with human immunodeficiency virus (HIV) infection have an increased risk of acute myocardial infarction (MI), and 6.5-15% of mortality in this population is attributable to cardiovascular disease. However, the angiographic pattern of coronary artery disease (CAD) in patients with HIV undergoing percutaneous coronary intervention (PCI) remains unknown. We sought to assess and describe the angiographic features and burden of CAD in patients with HIV as compared to those without HIV infection.
Methods and results: This is a retrospective, single-centre study comparing 93 patients with HIV infection who underwent PCI between 2003 and 2011 with 93 control patients without HIV infection matched for age (±3 years), gender, diabetes, and year of PCI (±2 years). Quantitative coronary angiography (QCA) was performed for all treated lesions at baseline and following PCI in both groups. One-year clinical outcomes post PCI were also analysed and compared. The mean age for both study populations was 57 years; patients with HIV were more likely to present with ST-segment elevation myocardial infarction (STEMI). Patients had a similar extent of CAD as measured by the presence of multivessel disease as well as SYNTAX score; however, patients with HIV were more likely to have lesions in the proximal segment of the respective coronary artery. While both groups mostly displayed none/mild calcified lesions, HIV+ patients had longer and fewer stenotic lesions. Clinical outcomes at one year were similar.
Conclusions: While HIV+ patients were more likely to present with STEMI, detailed coronary angiographic analysis revealed less complex lesions and favourable anatomy. This paradox may suggest alterations in genesis and progression of atherosclerosis in this clinical setting.
Introduction
The introduction of highly active antiretroviral (HAART) therapy in 1995 resulted in dramatic improvements in the prognosis of patients with human immunodeficiency virus (HIV) infection. Accordingly, the treatment paradigm in HIV has shifted to that of a chronic disease, with an increased attention to the characterisation and impact of HIV-associated vasculopathy, including cardiovascular atherosclerosis1-3.
Cardiovascular disease is currently the third leading cause of death among HIV-infected patients (HIV+), accounting for 6.5-15% of all deaths among this population. Furthermore, it is estimated that HIV confers a 50% increased risk of acute myocardial infarction (MI)4. Despite this, the angiographic pattern and burden of coronary artery disease (CAD) in HIV+ patients undergoing percutaneous coronary intervention (PCI) has not yet been clearly determined. Thus, we found it imperative that a comprehensive quantitative analysis be carried out to further our understanding of CAD in this unique population.
The purpose of the present study was to evaluate the angiographic morphology of CAD and assess possible correlations between angiographic morphology of CAD and clinical outcomes post PCI of HIV+ patients on HAART as compared to those without HIV infection (HIV–).
Methods
STUDY POPULATION
Patients who undergo cardiac catheterisation at Mount Sinai Hospital (NY, USA) are entered into a prospective database and are contacted at 30 days and one year following PCI via telephone calls. Among all patients who underwent cardiac catheterisation at our institution between January 2003 and December 2011, 279 patients were classified as immunocompromised in the database, including patients with either HIV infection or current malignancy or those who were under long-term use of steroids or immunosuppressive medications (i.e., patients with systemic lupus erythematosus or post-transplant patients). Out of 279 immunocompromised patients, 105 were identified as HIV+ based on the past medical history and the prescription of antiretroviral therapy through the Mount Sinai Data Warehouse, a secure, research information technology service which contains clinical data from hospital patient care processes. After excluding patients who had diagnostic angiography, plain old balloon angioplasty alone or failed PCI, our study population included 93 consecutive patients with known HIV infection who underwent PCI with at least one stent. The control group consisted of 93 HIV– subjects who underwent PCI during the same time period. While it is unknown whether those patients had ever been tested for HIV infection, no one was either under HAART treatment or listed as HIV infected as per their medical records, minimising the possibility of having an active HIV infection.
We compared baseline characteristics, procedural data, quantitative coronary angiography (QCA) and one-year clinical outcomes in HIV+ versus matched HIV– patients. Baseline characteristics, medications, and past medical history were obtained from medical chart review. All demographic and procedural data along with the results of quantitative coronary analysis were entered into a separate de-identified database. The study complied with the Declaration of Helsinki and was approved by the institutional review board (IRB).
QUANTITATIVE CORONARY ANGIOGRAPHY (QCA)
QCA analysis was conducted using validated quantitative angiographic software (QAngioXA 7.3; Medis medical imaging systems bv, Leiden, The Netherlands). It was performed for all treated lesions of study patients using baseline and post-PCI cineangiograms by two independent analysts from the Mount Sinai angiographic core lab blinded to patient HIV status and outcomes, with subsequent overreading by an experienced analyst.
QCA measurements included lesion length, lesion location, reference vessel diameter (RVD) (proximal, distal and interpolated), and percentage diameter stenosis (% DS) and minimal lumen diameter (MLD).
The TIMI grade flow and the corrected TIMI frame count (CTFC) were also assessed at baseline and post PCI as indicators of the coronary blood flow5,6. The SYNTAX score, which is a valid assessment of CAD burden and complexity, was calculated at baseline and post PCI (residual SYNTAX score) through the SYNTAX score calculator 2.117. Lesion calcification was classified as none/mild, moderate (radiopacities noted only during the cardiac cycle before contrast injection), and severe (radiopacities noted without cardiac motion before contrast injection compromising both sides of the arterial lumen)8. For bifurcation lesions, angiographic measurement was performed only for the main vessel. QCA was performed for all of the staged PCI procedures.
STUDY OUTCOMES
We report here one-year follow-up data. The composite endpoint of major adverse cardiac events (MACE), including death, myocardial infarction, recurrent coronary revascularisation (urgent or non-urgent), and stent thrombosis, was the primary endpoint of the study. Deaths were ascertained via the social security death index. Outcomes were then adjudicated by an independent clinical events committee, which included general and interventional cardiologists who were blinded to the patients’ HIV status, using available source documents.
STUDY DEFINITIONS
MACE were the composite of death, myocardial infarction (MI), and target vessel revascularisation (TVR). Death included all-cause mortality. Spontaneous and periprocedural myocardial infarction was defined according to the third universal definition of myocardial infarction9. Target lesion revascularisation (TLR) included clinically driven revascularisation including any percutaneous or surgical revascularisation due to ≥50% stenosis on angiography either within the stented segment or within the 5 mm segment proximal or distal to the stent edge. TVR included any clinically driven surgical or percutaneous intervention at any lesion located in a previously treated vessel. Stent thrombosis (ST) was defined using the Academic Research Consortium definition10. Multivessel coronary artery disease was defined as the presence of ≥50% luminal stenosis in two or more vessels.
STATISTICAL ANALYSIS
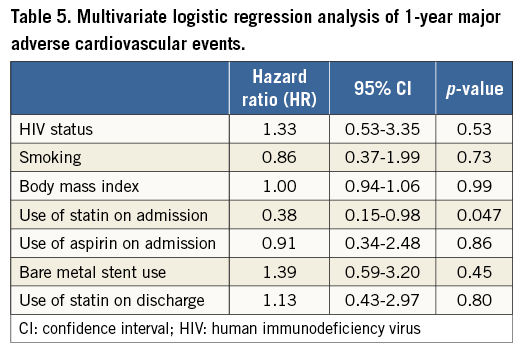
We performed a matched cohort study of HIV+ and HIV– patients matched for age (±2 years), gender, diabetes, and year of PCI (±3 years). Continuous variables are presented as mean±standard deviation or median (interquartile range) and compared using an unpaired Student’s t-test or the Wilcoxon rank-sum test, respectively. Categorical variables are presented as frequency (percentage) and compared using Pearson’s chi-squared test. Time-to-event analyses for HIV+ versus HIV– patients were performed using Kaplan-Meier methodology and compared using the log-rank test. Cox regression analysis was used to determine the hazard ratio (HR) for the one-year MACE. To assess the independent effect of HIV status on one-year MACE, multivariate regression analysis was used. Smoking status, body mass index (BMI), use of statins on admission, use of aspirin on admission, and use of bare metal stents were used for our multivariate analysis. P-values <0.05 were considered statistically significant. All statistical analyses were performed using STATA version 12.1 (StataCorp LP, College Station, TX, USA).
Results
Out of 186 patients who underwent successful PCI, 93 consecutive HIV+ patients and a control group of 93 HIV– patients were matched for age, gender, diabetes, and year of PCI. Mean age was 57 years (Table 1). Patients were predominantly male (77.4%) and mostly non-Caucasian (70%). HIV+ patients were more often smokers (60% vs. 22%, p<0.001), more likely to present with ST-segment elevation MI (STEMI) (12.9% vs. 2.2%, p=0.005), and had higher baseline C-reactive protein (CRP) values (7.5±9.1 vs. 4.3±6.4, p=0.008). Conventional cardiovascular risk factors were highly prevalent in both groups, but HIV– patients had higher BMI (27.8±6.6 vs. 30.4±6.6, p=0.009) and were more likely to have dyslipidaemia (88.2% vs. 95.6%, p=0.049). While LDL and HDL cholesterol did not differ significantly between the two groups, HIV+ patients had higher triglyceride levels (169±145 vs. 132±111, p=0.05).
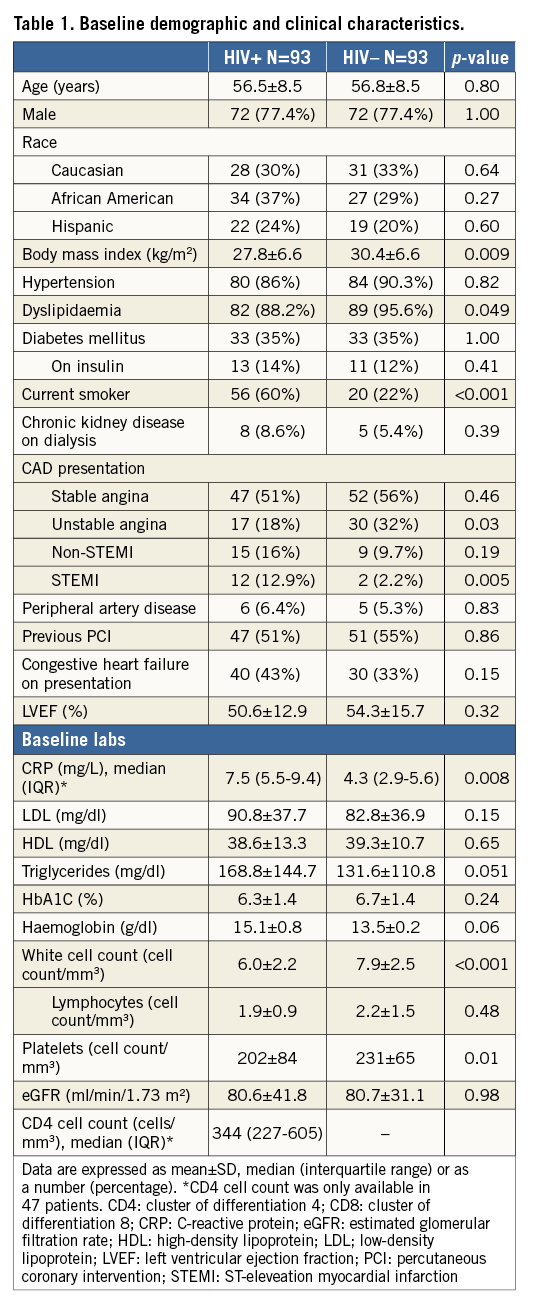
Table 2 shows the medications on admission and on discharge. The prescription of evidence-based medications on discharge was similar in the two groups with the exception of statins, which was considerably lower among HIV+ patients (63% vs. 84%, p=0.002). With regard to admission medications, statins (51% vs. 73%, p=0.002) and aspirin (78.5% vs. 90%, p=0.03) were less often prescribed in HIV+ patients. While information regarding HAART therapy including adherence or duration was not available, all patients with HIV were receiving antiretroviral therapy, which is consistent with the fact that the median CD4 cell counts in our cohort was greater than 200/mm3. Non-nucleoside reverse transcriptase inhibitors (NNRTI) and nucleoside reverse transcriptase inhibitors (NRTIs) were the most prevalent antiretrovirals (75% and 57%, respectively).
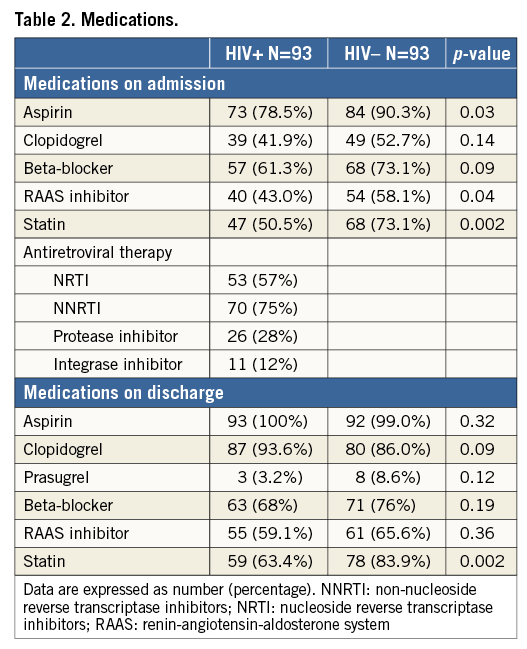
QUANTITATIVE CORONARY ANGIOGRAPHY
Angiographic characteristics are shown in Table 3. As many patients had more than one treated lesion, 144 treated lesions were analysed for the HIV+ group and 140 lesions for the HIV– group. Overall, HIV+ patients were found to have multivessel disease of mild complexity (mean SYNTAX score of 12) and their lesions displayed none/mild calcification pattern (63%), moderate lesion length (mean length of 17 mm) and mild obstruction (mean diameter stenosis of 68%). As compared to the control group, non-significant differences were detected with regard to multivessel disease (57% vs. 53%, p=0.22) and SYNTAX score (11.9±7.4 vs. 10.6±7.3, p=0.25). The arterial distribution of lesions was also similar; however, HIV+ patients were more likely to have proximal lesions. Accordingly, the reference vessel diameter was higher among HIV+ patients (2.65±0.70 mm vs. 2.34±0.63 mm, p=0.002). HIV+ patients were more likely to have lower diameter stenosis (67±15% vs. 71±16.5%, p=0.04) and nominally longer lesions (17.1±11 mm vs. 14.9±8.4 mm, p=0.07).
The two groups shared similar patterns of lesion calcification with none/mild calcification being the most prevalent pattern (63.4% vs. 61.8%, p=0.42). The baseline Thrombolysis In Myocardial Infarction (TIMI) grade flow was more likely to be 0/1 among HIV+ patients (15.2% vs. 10%, p=0.01), but final TIMI 3 flow was similar in the two groups (72% vs. 65%). The residual diameter stenosis post PCI was greater in HIV+ patients (18.9±8.4% vs. 16.4±8.5%, p=0.01). Lesions among HIV+ patients were more often treated with bare metal rather than drug-eluting stents (35.4% vs. 12.4%, p<0.001).
CLINICAL OUTCOMES
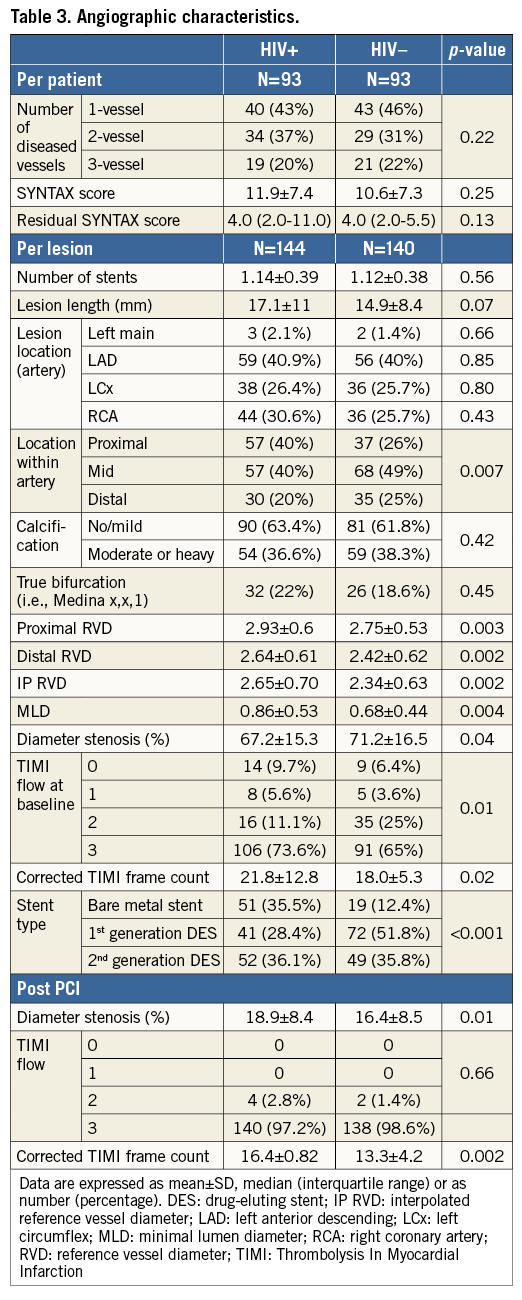
HIV+ patients had a higher one-year death rate (9.7% vs. 4.3%, p=0.045) and fivefold higher incidence of MI (5.4% vs. 1.1%, p=0.053). One-year TVR and TLR event rates were nominally higher among HIV+ as compared to HIV– patients (9.7% vs. 7.5%, p=0.56, and 4.3% vs. 3.2%, p=0.50, respectively) (Table 4). One-year MACE was similarly high in both groups (17.2% vs. 11.8%, p=0.13) (Table 4, Figure 1). Multivariate adjustment for major independent factors, including smoking status, BMI, use of statins on admission, use of aspirin on admission, and use of bare metal stents, did not alter clinical outcomes (Table 5).
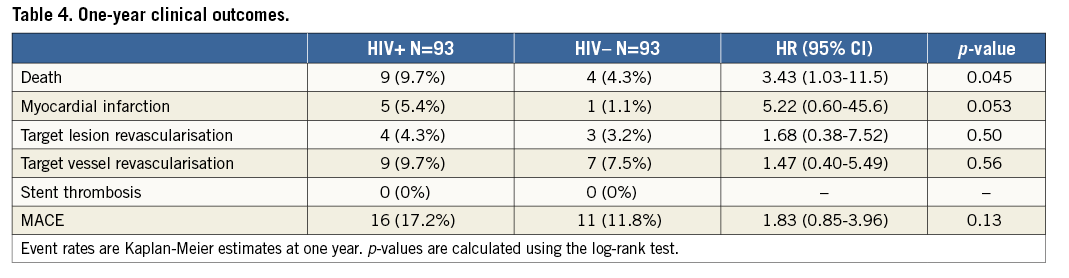
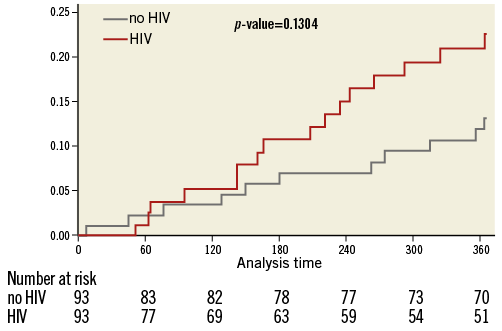
Figure 1. Rates of major adverse cardiac events (MACE) at 1 year. Kaplan-Meier curve showing the 1-year MACE rates between HIV positive and matched control patients undergoing PCI.
Discussion
To our knowledge, this is the first known study to examine angiographic features in HIV+ patients and possible correlations between the angiographic profile and clinical outcomes in this study population. Our study had several interesting findings. (1) The prescription rate of statin therapy was considerably lower in HIV+ patients both on admission and at discharge. (2) HIV+ patients had, overall, a high amount of multivessel disease with mild CAD burden, and their lesions were, for the most part, non/mild calcified, of moderate length and mildly obstructive. In comparison to HIV– patients, HIV+ lesions were characterised by a lower degree of diameter stenosis, localised in more proximal coronary segments and were marginally longer. (3) Despite their observed low-risk angiographic lesion profile, HIV+ patients were more likely to present with STEMI.
It is known that atherosclerosis in HIV+ patients is a multifactorial and complex process that involves chronic inflammation, immune dysregulation, coagulation disorders, and cardiometabolic abnormalities11-14. Furthermore, the prevalence of the conventional cardiovascular risk factors such as smoking, dyslipidaemia, diabetes, and hypertension in this population is higher compared to age-related controls15. Other known factors include side effects of HAART therapy (especially protease inhibitors and nucleoside reverse transcriptase inhibitors), recreational drug abuse (e.g., cocaine), and opportunistic infections that may contribute to premature and progressive atherosclerosis and thrombosis in these patients16-18.
Given the above unique clinical features of HIV infection and their associated high cardiovascular risk, we attempted to match our two groups in order to eliminate possible confounders in our study findings. As such, after matching for age, gender and diabetes, our study control group of HIV– patients was of a high-risk cardiovascular profile, as reflected by this population’s relatively young age and the increased prevalence of hypertension, dyslipidaemia and obesity.
While HIV infection and HAART therapy are well associated with metabolic abnormalities, our control group consisted mostly of obese patients (mean BMI >30), which may explain the high prevalence of cardiovascular risk factors in a relatively young population. HIV+ patients in our study were significantly more likely to present with MI, suggesting an increased HIV-related risk beyond traditional cardiovascular risk factors. This is consistent with the results of a recent large cohort study of 82,549 patients showing a 50% higher risk of acute MI in HIV+ patients as compared to HIV– after adjustment for coexisting comorbidities4.
A recent double-blind, randomised placebo trial compared atorvastatin to placebo in HIV+ patients with subclinical atherosclerosis in terms of aortic inflammation and coronary atherosclerotic progression. Atorvastatin was associated with a significant reduction in coronary plaque volume and high-risk coronary plaque features including positive remodelling19. Despite the established role of statins in primary and secondary prevention of cardiovascular risk20, HIV+ patients were paradoxically less likely to be on statin therapy both on admission and at discharge. This underuse of statin therapy might partially explain the higher incidence of MI in HIV+ patients. Concerns regarding potential drug interactions between statins and HAART therapy or hepatotoxicity may be the driving factors for lower use of statins in HIV+ patients21,22. Despite concerns regarding the possible interactions between statins and antiretroviral therapies, a series of studies has demonstrated the safety of atorvastatin, rosuvastatin and pitavastatin in this clinical setting23. Among HIV patients on a ritonavir/darunavir regimen, pitavastatin has been found to be safe and effective with minimal drug-to-drug interactions24. Whether pitavastatin can lead to decreased cardiovascular events in HIV patients is under ongoing investigation (Evaluating the Use of Pitavastatin to Reduce the Risk of Cardiovascular Disease in HIV-Infected Adults – REPRIEVER study).
ANGIOGRAPHIC ANALYSIS
Our QCA analysis provides the first known angiographic description of coronary vasculopathy and lesion characteristics in patients with HIV. Overall, the extent of CAD was similar in the two groups. Our data appear to confirm the findings of previous studies in which HIV+ patients displayed a similar CAD burden to age-matched control patients25,26. While previous reports assessed the extent of CAD based on the number of diseased vessels alone, we further investigated by quantifying the CAD burden with the SYNTAX score. The two groups displayed less complex coronary lesions and favourable anatomy as assessed by the SYNTAX score. Nevertheless, a detailed analysis of lesion characteristics revealed notable angiographic differences between the two groups that had not been previously described. Such differences include disparities in vessel diameter, lesion location, length, obstruction grade, and the impact of those lesions on TIMI flow rate.
Recent data have shown a positive correlation between HIV infection and positive arterial remodelling that further leads to an increased risk for myocardial infarction27. Our QCA findings of HIV lesions, including greater vessel lumen diameter, milder obstruction, more proximal and none/mild calcified lesions, suggest a high risk for ruptured plaques and may provide indirect evidence of positive remodelling in this cohort.
The aforementioned angiographic differences and the apparent discord between angiographic profile and clinical presentation seen in HIV+ patients may reflect substantial differences in the pathogenesis of atherosclerosis in HIV+ patients (Figure 2). In vivo intravascular imaging studies with intravascular ultrasound as well as optical coherence tomography may further elucidate the differences in atherosclerosis features in patients with HIV as compared to those without HIV.
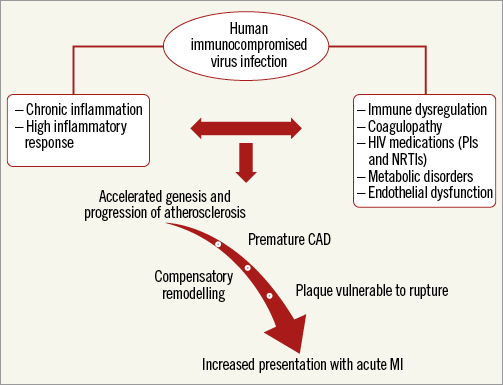
Figure 2. Pathogenesis of coronary artery disease in HIV patients (proposed). A proposed flow chart schema indicating the pathogenesis of coronary artery disease in HIV patients.
As it relates to PCI characteristics, we observed a higher frequency of bare metal stent use in HIV+ patients that may be attributable to clinical and environmental characteristics, raising concerns about long-term dual antiplatelet therapy in this population (e.g., risks of bleeding, non-compliance). The use of bare metal stents in this patient population would not be supported by the most recent evidence28,29. However, the extended window of inclusion of patients in the present study (i.e., 2003-2011) may explain, at least in part, the high use of bare metal stents in the study population.
CLINICAL OUTCOMES
Previous studies have reported similar rates of both stent thrombosis and clinical restenosis among HIV+ and HIV– patients following PCI, although HIV was associated with an increased risk of MI30-32. These data were corroborated by a recent large meta-analysis of 2,440 HIV+ patients showing that those patients presenting with ACS had a significant long-term risk of MI30. Similarly, we observed that HIV+ patients had a trend to a higher incidence of MI compared to our matched HIV– patients, with no significant differences in terms of repeat revascularisation and stent thrombosis. With respect to MI, out of five such incidents in HIV+ patients, two were periprocedural and three were spontaneous, whereas HIV– patients developed only spontaneous MI. Based on our findings, the association between HIV infection and MI may be explained by the HIV infection itself, disparities in statin use, and differences in lesion characteristics between the two groups.
Although not significantly different compared to the control population, the one-year MACE incidence in HIV+ patients was as high as 17%, underscoring the importance of implementing aggressive preventive strategies in this high-risk patient population.
Limitations
The present study had several limitations. Firstly, our analysis was retrospective and of a single-centre patient population. Secondly, we did not have access to information regarding the duration of HIV infection, HAART therapy, and viral load, and only had CD4 cell counts available in a subset of HIV+ patients. Finally, we included patients undergoing PCI between 2003 and 2011, with potential treatment biases related to changes in PCI practice during this timeframe.
Conclusions
In conclusion, our QCA analysis suggests that HIV+ patients on HAART therapy undergoing PCI display considerable differences in their lesion profile. Furthermore, quite interestingly, our findings indicate that HIV+ patients exhibiting benign angiographic characteristics still presented more often with STEMI, suggesting possible altered genesis and progression of atherosclerosis in the setting of HIV infection. The clinical complexity and angiographic pattern of HIV patients warrants further investigation through observational studies and implementation of more effective preventive strategies in this high-risk patient population.
| Impact on daily practice HIV patients undergoing PCI are relatively young, have complex metabolic profiles, and more frequently present with STEMI. Paradoxically, HIV patients seem to be less often on statins, probably due to physicians’ concerns regarding possible drug interactions between HAART and statins. Angiographic analysis reveals a benign angiographic profile with milder obstruction, more proximal, and none/mild calcified lesions compared to a matched control group. The discordance between angiographic phenotype and clinical presentation suggests possible altered genesis and progression of atherosclerosis in the setting of HIV infection. The clinical complexity and angiographic pattern of HIV patients warrant further investigation and the implementation of more effective and aggressive preventive strategies in this high-risk patient population. |
Acknowledgement
All authors have read and approved the manuscript prior to submission. Each author has contributed significantly to the manuscript including: (1) substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data, (2) drafting the article, revising it critically for important intellectual content, and (3) final approval of the version to be published.
Conflict of interest statement
R. Mehran has received institutional research grant support from The Medicines Company, Bristol-Myers Squibb/Sanofi-Aventis and Lilly/Daiichi Sankyo, and consulting fees from Abbott Vascular, AstraZeneca, Boston Scientific, Covidien, CSL Behring, Janssen Pharmaceuticals, Maya Medical, Merck and Regado Biosciences. G. Dangas serves on the advisory board of and has received lecture honoraria from Bristol-Myers Squibb/Sanofi-Aventis. P. Moreno serves on the Scientific Advisory Board and on the speakers’ bureau for AstraZeneca. A. Kini serves on the speakers’ bureau of the ACC and has received consulting fees from WebMD. S.K. Sharma serves on the speakers’ bureau of Abbott Vascular, AngioScore, Boston Scientific, Lilly/Daiichi Sankyo and The Medicines Company. The other authors have no conflicts of interest to declare.

