Introduction
The majority of acute coronary syndromes (ACS) are caused by the rupture or erosion of coronary atherosclerotic plaques. In patients with myocardial infarction (MI), recurrent events tend to accrue despite the implementation of secondary prevention measures, which mainly consist of pharmacotherapy. During the last decade, advances in intravascular imaging (i.e., intravascular ultrasound [IVUS], optical coherence tomography [OCT], and near-infrared spectroscopy [NIRS]) led to the identification of morphological features that define “vulnerable plaques” and are linked to higher rates of cardiovascular events. It has been hypothesised that preventive stenting might passivate these lesions, preventing the occurrence of plaque-related acute coronary syndromes. However, stenting can be also associated with adverse outcomes, and no solid evidence is currently available on its use in this sort of “primary prevention” setting. As such, the optimal management of vulnerable plaques has not been established so far and is currently a matter of debate.
Pros
Duk-Woo Park, MD, PhD; Hoyun Kim, MD
Thrombosis of lipid-rich thin-capped atherosclerotic lesions (“vulnerable plaques” [VP]) is the cause of most ACS and unexpected sudden cardiac deaths1. VP are often non-flow limiting, but they can be identified with intravascular imaging. With the advancement of multiple intracoronary imaging modalities, including IVUS, virtual histology IVUS (VH-IVUS), OCT and NIRS, several specific, high-risk morphological characteristics of VP, such as large plaque burden, small minimum lumen area (MLA), lipid-rich fibroatheroma, and thin-cap fibroatheroma (TCFA), have been identified. Although VP are prone to unanticipated ACS or acute coronary events, there is no consensus on the optimal management of VP; guideline-directed medical therapy is generally recommended for patients with atherosclerotic VP. However, given the fact that recurrent adverse cardiovascular events still occur despite optimal medical treatment (OMT), there are unmet needs for the further management of VP. Theoretically, this prompts an increasing interest in local preventive therapy (i.e., percutaneous coronary intervention [PCI] with stenting) as a potential therapeutic strategy, which may seal and passivate VP, preventing future coronary events.
Although current evidence is still lacking on the benefit of local preventive PCI on VP compared with OMT alone, there are several reasons why local preventive PCI could improve clinical outcomes in patients with vulnerable coronary plaques. Several prospective, observational studies showed that a significant proportion (approximately 10%) of patients presenting with ACS who had undergone PCI of the culprit lesion experienced recurrent major adverse cardiovascular events due to non-culprit VP, mostly rehospitalisation for unstable or progressive angina and MI23. Therefore, prophylactic PCI with stenting could help prevent spontaneous MI and reduce hospitalisations due to unstable or progressive angina. In addition, a pilot randomised trial has demonstrated that PCI may safely treat these lesions, enlarge the lumen, and thicken the overlying fibrous cap at 2-year follow-up4. With the current advanced intravascular imaging modalities that can more specifically identify VP, a plaque-centred, tailored, pre-emptive strategy that includes prophylactic stenting could be theoretically beneficial. However, the efficacy of localised preventive therapy with PCI treatment of VP in reducing adverse cardiac events, as compared with OMT alone, has never been studied in a randomised trial powered for clinical events.
The Preventive PCI or Medical Therapy Alone for Vulnerable Atherosclerotic Coronary Plaque (PREVENT) Trial is a multicentre, open-label, active treatment-controlled randomised trial comparing preventive PCI plus OMT versus OMT alone in more than 1,600 patients with functionally insignificant (fractional flow reserve [FFR]>0.80) VP containing multiple high-risk features (Figure 1)5. On IVUS, OCT, VH-IVUS or NIRS-IVUS, VP had at least 2 of the following 4 characteristics present: TCFA (OCT or VH-IVUS); MLA ≤4.0 mm2 (IVUS); plaque burden at the MLA site >70% (IVUS); or lipid-rich plaque (maximum lipid-core burden index [LCBI]4mm >315) (NIRS). The primary outcome was the composite of death from cardiac causes, target vessel MI, ischaemia-driven target vessel revascularisation, or hospitalisation for unstable or progressive angina at 2 years. The upcoming results of the PREVENT Trial are expected to be available in early 2024.
In summary, vulnerable coronary plaques have been identified with the advancement of intravascular imaging and can cause recurrent adverse cardiovascular events despite OMT. Therefore, as a local preventive therapy, preventive PCI for VP could be an option to improve clinical outcomes. The PREVENT Trial is the first randomised trial of preventive PCI for the treatment of non-flow-limiting VP in patients with coronary artery disease (CAD) that has been powered for improved clinical outcomes. The results will be available soon.
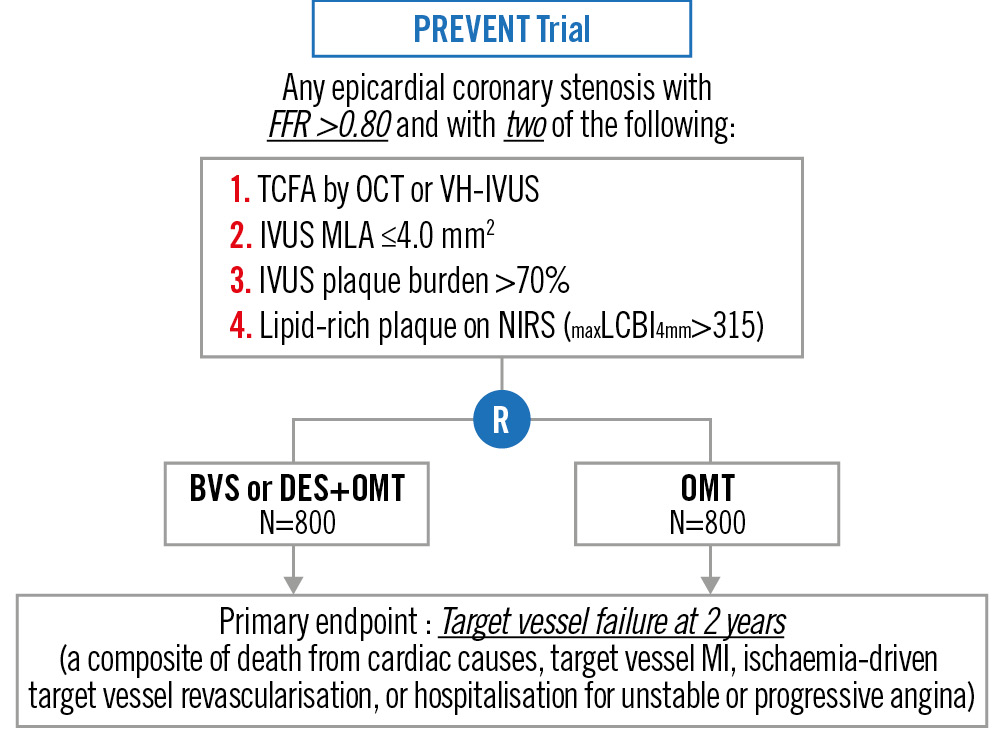
Figure 1. Overview of the PREVENT Trial BVS: bioresorbable vascular scaffold; DES: drug-eluting stent; FFR: fractional flow reserve; IVUS: intravascular ultrasound; LCBI: lipid-core burden index; MI: myocardial infarction; MLA: minimum lumen area; NIRS: near-infrared spectroscopy; OCT: optical coherence tomography; OMT: optimal medical therapy; TCFA: thin-cap fibroatheroma; VH-IVUS: virtual histology intravascular ultrasound
CONS
David L. Brown, MD; Ayesha Singh, MD
Since almost all type 1 MIs are precipitated by thrombosis at the site of a disrupted atherosclerotic plaque, there has been great interest in prospectively identifying the features of atherosclerotic plaque that make it vulnerable to disruption in the future. Post-mortem analyses of plaques that have undergone recent disruption have revealed consistent findings, including a thin fibrous cap, positive remodelling, and a large necrotic core, often found together and termed a TCFA6. VP characteristics can be demonstrated prospectively in vivo by IVUS with VH, OCT, or coronary computed tomography angiography (CCTA). The ability to prospectively identify the structural signature of VP has led some experts to propose the implantation of a stent in the hope of “stabilising” the VP. In theory, such a strategy would prevent future plaque disruption events at that site and thereby reduce acute MI and its associated morbidity and mortality. However, we believe there is insufficient understanding of both the biology of VP and the risk-benefit ratio of such an endeavour to embark on prophylactic coronary artery stenting for the primary prevention of acute MI.
Atherosclerosis is a chronic inflammatory process that results in the development of VP in the coronary arteries. However, at any point in time, local proinflammatory processes are counterbalanced by anti-inflammatory processes, resulting in a dynamic situation where an image obtained at a single point in time is simply a snapshot of a constantly changing microenvironment7. The dynamic nature of VP has been documented with IVUS and VH8. Of 20 VP identified as TCFA, only 25% retained that morphology on repeat imaging 12 months later. The remainder healed by evolving into different lesion types not associated with an elevated risk of plaque disruption. This finding may explain why prospective analyses of VP have such a poor positive predictive value for plaque-specific clinical events. For example, in the PROSPECT trial, 596 TCFA were identified using IVUS, but only 26 major adverse cardiovascular events (4.3%) were related to TFCA during the 3 years of follow-up2. In the SCOT-HEART Trial, coronary plaques with high-risk characteristics (positive remodelling or low-attenuation plaque) were observed on CCTA in 608 patients, of whom only 25 experienced subsequent MI or coronary death (4.1%) after 4.7 years9. Similarly, in the PROMISE Trial, coronary plaques with adverse characteristics (positive remodelling, low CT attenuation, or napkin-ring sign) were observed on CCTA in 505 patients with non-obstructive CAD, of whom only 24 experienced an adverse cardiac event (4.8%) during a median follow-up of 25 months10. Of note, only the PROSPECT trial attempted to correlate downstream plaque rupture events with a prospectively identified VP. SCOT-HEART and PROMISE merely reported the association of future events with the presence of high-risk plaque characteristics but did not attempt to attribute these events to specific VP. Thus, in the best-case scenario, assuming all downstream events were proven to be caused by a prospectively identified VP, for every 100 patients who undergo stenting of a presumed VP, ~95 would derive no benefit. Furthermore, there is no reason to believe that patients undergoing intravascular imaging and/or stenting of VP would not experience acute procedural complications nor the annual 2% rate of cardiac death, MI, or ischaemia-driven target lesion revascularisation that has been documented in years 1-5 following stent placement and may persist in perpetuity11.
In summary, given the dynamic nature of the proposed target for stenting, the poor sensitivity and specificity of current imaging techniques, and the great potential for harm, there is simply not enough evidence to justify the stenting of individual VP. As atherosclerosis is a systemic and dynamic disease, we feel that the maximum benefit will be derived from systemic as opposed to focal treatments until prospective data from well-designed and adequately powered randomised clinical trials prove otherwise.
Conflict of interest statement
The authors have no conflicts of interest to declare.

