Atherosclerosis is one of the major causes of morbidity and mortality in developed countries. The major cause (86%) of cardiovascular death from heart attacks as well as the major cause (45%) of deaths from brain aneurysm are due to less obtrusive plaques known as vulnerable plaques that rupture suddenly and trigger a blood clot or thrombus that blocks blood flow1-4. Early detection of plaque lesions is the first and necessary step in preventing the lethal consequences of atherosclerosis4,5.
In order to investigate the morphology and severity of atherosclerotic lesions, imaging techniques such as angiography, CT angiography (CTA), and contrast-enhanced magnetic resonance angiography have been developed. However, accurate characterisation of a coronary atherosclerotic plaque and the diagnosis of latent vulnerability of a plaque lesion require an imaging modality that enables quantification of both plaque structure and plaque components of lipid-rich, fibrous, or calcified plaque. Intravascular ultrasound (IVUS) and optical coherence tomography (OCT) are currently the two most commonly used modalities in clinics for diagnosing atherosclerosis5.
IVUS has been used to demonstrate a positive correlation between cholesterol levels and percent atheroma volume5,6. Additionally, the development of virtual histology has aided the characterisation of plaque composition5,6. However, even though IVUS uses frequencies from 20 to 40 MHz that provide good penetration depth, it has insufficient accuracy (resolution 100 μm) to study thin-cap fibroatheroma (TCFA) lesions5,6. This was reinforced by recent research in porcine coronary arteries in which no correlation was found between the necrotic core sizes determined by real and virtual histology, questioning the role of IVUS in the detection of TCFA7. Higher resolution IVUS images can be obtained using a high-frequency ultrasound transducer, but this comes at the cost of limited penetration depth.
OCT provides real-time and noninvasive images with micrometre resolution, which can be up to two orders of magnitude smaller than that of IVUS8. OCT is characterised by excellent image resolution and has also been used to identify thin fibrous caps, the extent of necrotic cores, possible presence of macrophages in the fibrous cap as well as neo-intimal growth within stents5,9. However, OCT is restricted to imaging 1 mm to 2 mm below the surface of biological tissue. Therefore, using OCT to obtain information on the entire cross-sectional artery anatomy and to quantify the arterial thickness is difficult5.
IVUS and OCT provide complementary information for vascular imaging applications6,10. Integration of IVUS/OCT images combines the advantages of high-resolution imaging of OCT and broad imaging range of IVUS. Recently, Sawada et al studied the feasibility of the combined use of IVUS and OCT data (images acquired separately) for detecting TCFA in 56 patients6. The result shows that neither modality alone is sufficient for detecting TCFA. The combined use of OCT and IVUS is a more effective approach for evaluating TCFA6. However, so far no method has been reported on enabling the fusion of matched IVUS/OCT images, which is essential in order to take advantages of complementary information provided by IVUS and OCT.
In this issue of EuroIntervention, Räber et al describe a method to match IVUS and OCT images acquired separately11. The method is based on the identification of common landmarks, such as side branches, bifurcations or large calcifications. They demonstrated the feasibility of this method in three sections of coronary artery, showing various plaque morphologies from separate IVUS and OCT pullback scans obtained from patients. Based on the matched and fused images, an OCT/IVUS/VH tissue classification algorithm is proposed to take into account of the individual strength of both techniques. They demonstrated that off-line fusion of co-registered IVUS and OCT is feasible and combines the strengths of both imaging modalities, potentially improving the diagnostic accuracy of plaque characterisation.
However, as stated by the authors, the reported method has limitations. One limitation is that their fusion approach is based on manual identification and extraction of landmarks in both OCT and IVUS datasets. The fused image is accomplished by establishing correspondence between the image datasets, followed by aligning and fusing the two datasets. These procedures are slow and not automatic. Furthermore, these manual processing steps have the propensity to introduce bias and inaccuracy. In spite of these limitations, the authors clearly demonstrated that off-line fusion of co-registered IVUS and OCT is feasible and provides valuable information for improving the diagnostic accuracy of atherosclerosis. This research can serve as a preamble for the future development of an automatic algorithm to perform matching and fusion of IVUS/OCT images. Additionally, this off-line fusion demonstrates the potential usefulness of a combined IVUS/OCT device that would allow simultaneous image acquisition and online fusion of images. This is where future collaboration between doctors and engineers will be very important.
Currently, multimodal intracoronary imaging requires introducing separate imaging probes as described by the authors and other studies6. Current practice not only requires a laborious matching procedure for image co-registration and fusion, but it is also time consuming and costly. Therefore, it is significantly advantageous to integrate IVUS/OCT imaging into a single system. We have reported the development of an integrated intravascular imaging system that combines IVUS/OCT12-14. An integrated IVUS/OCT probe with an outer diameter of less than 0.7 mm has been developed13. Simultaneous in vitro IVUS/OCT images of a human coronary artery with calcified plaque are shown in Figure 1. The plaque can be identified in both OCT and IVUS images, and the fused IVUS/OCT image is shown in Figure 1C. Recently, we have also demonstrated in vivo images of vascular tissue in rabbit and porcine animal models using this integrated IVUS/OCT probe14. Although further research and development is required to translate this technology to clinical practices, the results clearly demonstrate the potential of integrated IVUS/OCT system.
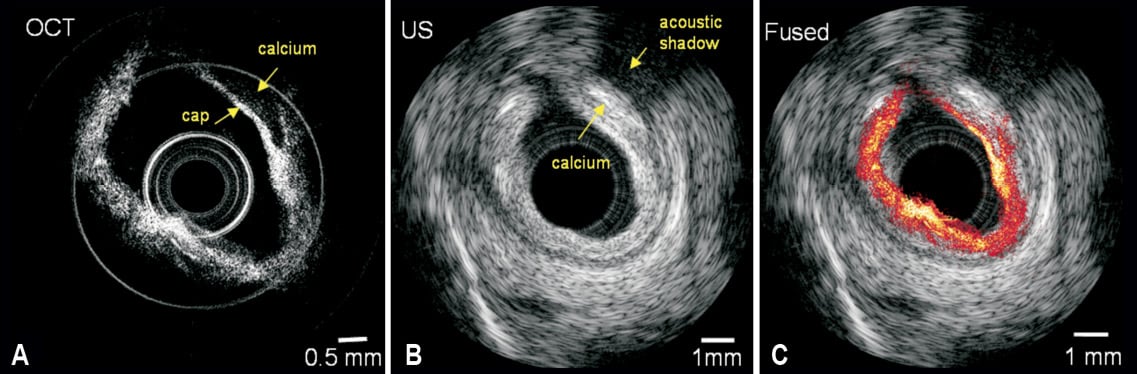
Figure 1. IVUS/OCT images of human coronary artery specimen from integrated OCT/US system. (A) OCT image, (B) ultrasound image, (C) fused OCT/US image. Adapted and reproduced from Yin et al13 with permission from J Biomed Opt.
In summary, histological studies have identified several characteristics of plaques that are classified as vulnerable and it is important to detect these plaques early and manage them before they become life threatening. Vulnerable plaques cannot be seen by traditional angiography, since most of these occurrences have a length scale of 2~150 microns. It is essential to develop a real-time high resolution imaging technology that can resolve vascular tissue elements in the artery at such scales. Current research from Räber et al demonstrates the potential of using an off-line fusion algorithm to investigate the power of combining information from IVUS/OCT for imaging and characterisation of vulnerable plaques, which will add tremendously to our clinical knowledge11. However, the development of simultaneous co-registration of IVUS/OCT images using a single bimodal in vivo imaging catheter would be critical for translation of this technology to clinical applications. It will require continued collaboration between physicians and engineers.
Conflict of interest statement
Z. Chen has a financial interest in OCT Medical Imaging Inc., which, however, did not provide support for this research. P.M. Patel has no conflict of interest to declare.

