
The evolution of coronary atherosclerosis is not a long quiet river, but rather follows a tumultuous flow. The growth of plaque is a dynamic phenomenon that is not only related to linear volume increase due to progressive lipid accumulation, fibrosis and inflammation but is also marked by episodes of sudden instability, mostly created by atherothrombotic acute episodes1. As a consequence, patients could alternate between long stable clinical periods and acute events, i.e., chronic and acute coronary syndromes (ACS)2. These latter events are essentially induced by symptomatic plaque rupture or erosion. However, pathology studies have strongly suggested that some plaques may rupture silently without causing symptoms, as they are frequently observed in native coronary arteries, even in the absence of any medical history of previous myocardial infarction or unstable angina3,4. Conventional angiography alone is limited for identifying these relics of the past, but intracoronary imaging techniques, intravascular ultrasound (IVUS) and especially optical coherence tomography (OCT, which has a higher spatial resolution), can accurately analyse vessel wall and atherosclerotic plaque structure and thus are tools which are adapted to achieving this goal, giving birth to a growing body of literature. Hence, Souteyrand and colleagues were the first to observe with serial OCT analyses the spontaneous healing process following plaque rupture or erosion in conservatively treated ACS patients: they identified the residual thrombotic material incorporated within the plaque and neointimal covering5.
In this issue of EuroIntervention, Wang and colleagues report the results of an intracoronary imaging analysis including 204 patients with stable angina or acute coronary syndrome for whom the culprit lesion was imaged by OCT and, in a minor subgroup, IVUS6.
The authors observed the presence of multilayered (ML) plaques (i.e., atherosclerotic plaques displaying distinct optical densities as a result of previous successive ruptures and healings) in 58% of the cases, including 75% of the stable and more than 50% of the unstable cases. These ML plaques were associated with longer lesions and more severe stenosis, according to angiographic and intracoronary imaging criteria, but surprisingly did not display a higher frequency of the classic features of vulnerable plaque such as thin fibrous cap, large lipid pool or microchannels compared to the non-ML lesions. Moreover, detection of ML plaque on the culprit site did not significantly impact on outcome, as the one-year incidence of severe adverse clinical events and the need for repeat revascularisation were comparable between groups. Thus, these results suggested that, despite a worse angiographic presentation, ML plaques were not associated with a worse clinical prognosis.
These data are remarkably in line with former pathological observations that were the first to highlight the high frequency of the phenomenon. Burke and colleagues reported the presence of healed plaques in 61% of their series of patients with sudden cardiac death. These features were found in 68% of the stable plaques and 52% of the unstable (ruptured or eroded) plaques3. Furthermore, Mann and Davies also observed that ruptured/healed plaques were more frequently identified in tighter coronary lesions (>51% diameter stenosis ) compared to intermediate (<50% diameter stenosis) lesions and concluded that this phenomenon was a significant substrate for atherosclerotic plaque progression4. However, ML plaques do not appear to be as frequently observed among culprit lesions in ACS as they are in chronic coronary syndrome patients. Previous series published earlier this year identified ML features within the culprit lesion only in 28.7% of conventional ACS7 and 3.3% of recurrent ACS subjects (≥3 acute myocardial infarction or ≥4 ACS including one acute MI)8. Interestingly, Fracassi and colleagues found, like Wang et al, that ML plaques were associated with more complex lesions and a higher degree of stenosis, but they also observed a higher incidence of local inflammation signs (thin fibrous cap, local macrophage infiltration) in ML compared to regular non-ML plaques7. Furthermore, these authors reported, once again like Wang et al, that identification of ML morphology at the culprit lesion site did not carry an adverse prognosis in terms of major adverse cardiovascular events7.
Altogether, the intracoronary imaging and pathology data suggest that rupture or erosion of the atherosclerotic plaque could evolve in two distinct directions (Figure 1). The first path is the occlusion/subocclusion of the arterial lumen by a major thrombosis, leading to symptomatic ACS. The second path, that appears to be rather common, is the in situ spontaneous plaque healing without reported symptoms. In this latter scenario, the destabilisation/cicatrisation process (including thrombus and surrounding tissues) could potentially repeat over time and end in a “quiescent” stable lesion that would display greater remodelling and a smaller vascular lumen5. Plaque rupture and erosion are both largely underlaid by inflammatory phenomena9. Macrophage-synthesised protease enzymes degrading all components of the arterial extracellular matrix, lymphocyte-secreted interleukins and cytokines, modification in local shear stress, and cholesterol crystal accumulation are known to promote plaque rupture, whereas neutrophil activation, smooth muscle cell migration and endothelial cell apoptosis are involved in plaque erosion9. However, both phenomena could eventually lead to thrombosis but also frequently to healing. The reasons why a vulnerable plaque could spontaneously pacify and stop its progression towards a marked atherothrombotic event remain, to this date, largely unknown. However, unravelling these mechanisms would set up an important milestone in the quest for more efficient anti-atherosclerosis targeted therapy.
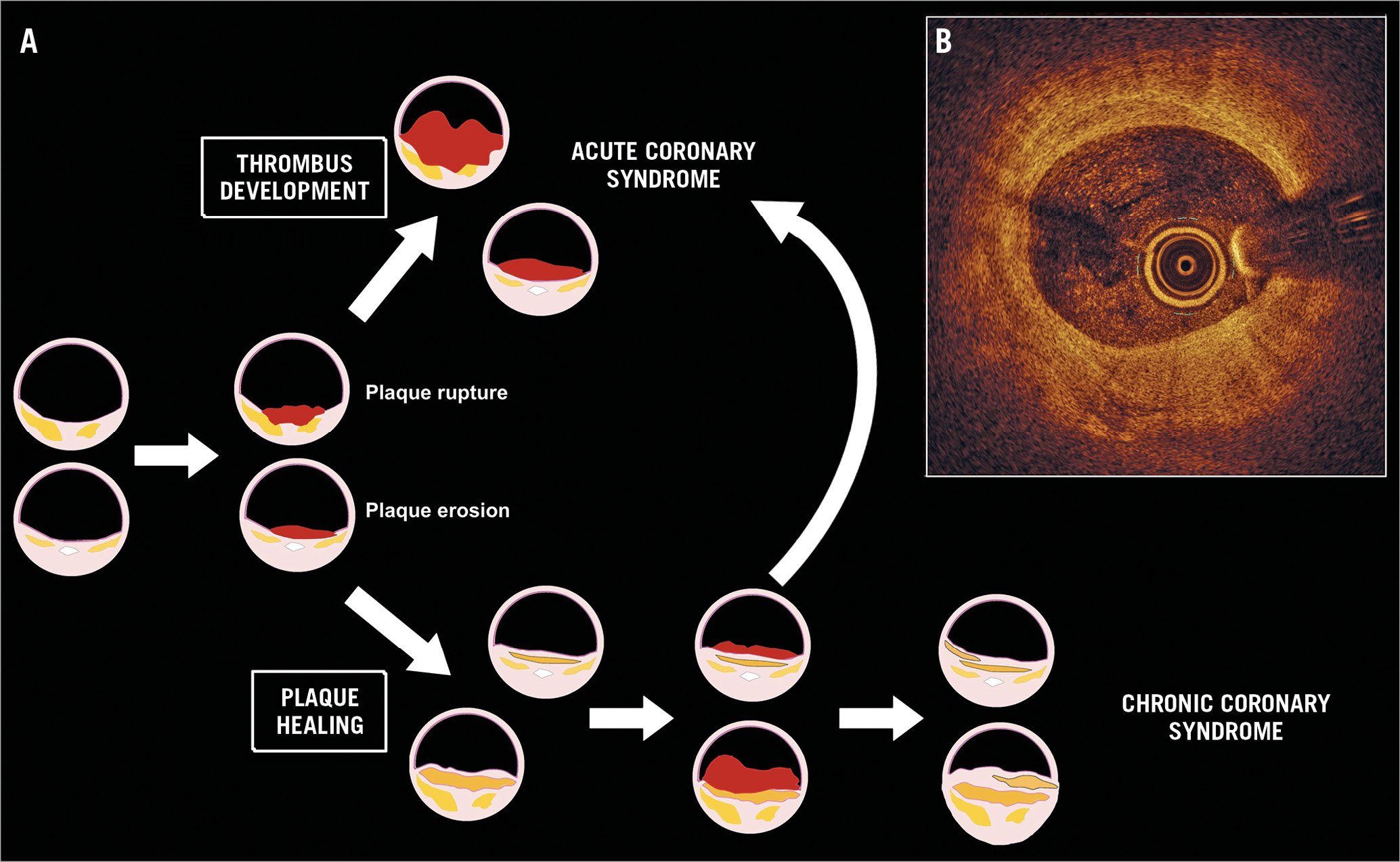
Figure 1. The flow of atherosclerotic plaques. A) Once it is eroded or ruptured, the atherosclerotic plaque is overlaid by a thrombus that could grow and create the substrate for symptomatic ST- or non-ST-elevation acute coronary syndromes (upper part of the Figure). However, the plaque can also spontaneously heal and stabilise (lower part of the Figure). Sequential rupture/healing cycles are possible that could create the substrate for a subsequent acute atherothrombotic event or silently contribute to the development of a chronic coronary syndrome. The cicatricial tissue is incorporated within the lesion, increasing the global plaque burden and leading to progressive arterial lumen narrowing. B) An example of a multilayered atherosclerotic plaque identified by OCT analysis in a 65-year-old man with no previous history of coronary artery disease. The image displays distinct areas with different optical densities that bear witness to the presence of cicatricial tissue, lipid pools, macrophage infiltration and punctiform calcifications.
Conflict of interest statement
N. Amabile has received a research grant from Abbott Vascular, proctoring and consulting fees from Abbott Vascular and Boston Scientific, and consulting fees from Biosensors. A. Veugeois has no conflicts of interest to declare.

