
Abstract
Aims: The aim of this study was to determine the prevalence and significance of plaque with a multilayered (ML) pattern in patients with acute coronary syndrome (ACS) versus stable angina pectoris (SAP) using OCT.
Methods and results: Two hundred and four patients (144 ACS and 60 SAP) with OCT imaging of the culprit lesions before intervention were studied. ML plaques were identified by OCT as plaque with multiple layers of distinct optical signals. ML plaque was identified in 119 out of 204 (58.3%) patients. ML plaques were more frequently observed in SAP than ACS (75% vs 51.4%, p=0.001). Patients with prior myocardial infarction (MI) had a higher incidence of ML plaque compared with those without (74.4% vs 54.5%, p=0.024). ML plaque had a higher degree of luminal stenosis (p=0.006), longer lesion length (p=0.025), more complex lesion type (B2/C) (p<0.001) on angiography and non-significant larger plaque burden (p=0.07) on IVUS compared with those without an ML pattern.
Conclusions: ML plaques, indicative of prior thrombosis, were frequently identified in patients with CAD, particularly more so in SAP and those with prior MI compared with ACS. The presence of an ML pattern is a marker of a greater extent and severity of CAD, suggesting a pathogenic link between plaque healing and lesion progression.
Introduction
Plaque rupture and plaque erosion are believed to be the most common underlying mechanisms for acute coronary syndrome (ACS)1,2. However, not all plaque ruptures and erosions lead to acute coronary events3,4. In contrast, these abrupt changes are often clinically silent but may contribute to rapid plaque progression and the development of high-degree stenosis3,5. Pathologically, healed ruptures/erosions often exhibit a multilayered (ML) pattern composed of distinct layers of collagen2. The prevalence and detailed morphological features of healed plaques, however, have never been investigated in vivo. Optical coherence tomography (OCT) with high resolution enables visualisation of the coronary arterial wall microstructure, specifically superficial tissue components1,6,7. As plaques with an ML pattern are composed of distinct tissue components with unique optical characteristics, OCT can be used to detect these multiple layers which are the pathologic hallmark of healed plaques6. The primary goal of this study was to determine the prevalence, morphological features, and significance of ML plaques as detected by OCT in patients with ACS and stable angina pectoris (SAP).
Methods
STUDY POPULATION
A total of 268 consecutive patients with ACS or SAP who underwent pre-interventional OCT imaging of the culprit lesions were included. Sixty-four patients were excluded for the following reasons: prior stenting (n=23), significant residual thrombus (n=7), poor image quality (n=11), incomplete culprit lesion imaging (n=5), and severe calcification (n=10). In addition, eight patients with ACS caused by coronary spasm, spontaneous coronary dissection, or other unclassified lesions were also excluded. A total of 204 patients (144 ACS and 60 SAP) with analysable OCT images were included in the final analysis. In patients with ACS (64 ST-segment elevation myocardial infarction [STEMI] and 80 non-ST-segment elevation acute coronary syndrome [NSTEACS]), aspiration thrombectomy (Export® aspiration catheter; Medtronic, Santa Rosa, CA, USA) prior to OCT imaging was allowed in patients with large occlusive thrombus or a Thrombolysis In Myocardial Infarction (TIMI) flow grade <2. All treatment strategies were decided by the operators according to local practice. Intravascular ultrasound (IVUS) was performed in a subset of 45 patients. The study was approved by the institutional review boards and all subjects provided informed consent.
OCT AND IVUS IMAGE ACQUISITION AND INTERPRETATION
After diagnostic angiography, IVUS and OCT imaging were performed in the culprit vessel. IVUS examinations were performed using an Atlantis™ Pro catheter (Boston Scientific, Marlborough, MA, USA) after intracoronary administration of 100 to 200 µg nitroglycerine. The catheter was pulled back automatically at 0.5 mm/s. Following IVUS imaging, OCT imaging was performed as previously described1.
OCT images were analysed using an offline review workstation.
An ML pattern was defined as multiple layers with distinct optical density from underlying plaque components according to previous ex vivo and pathology validation studies (Figure 1, Figure 2),6,8. Fibrous or lipid was defined as previously reported7,9. Fibrous cap thickness (FCT) was measured three times at its thinnest part, and the average value was calculated. Thin-cap fibroatheroma (TCFA) was defined as a plaque with lipid content in at least two quadrants, with the thinnest part of the fibrous cap measuring less than 65 µm10. Plaque rupture was identified by the presence of fibrous cap discontinuity with a clear cavity formed inside the plaque1,11,12. Plaque erosion was defined as the presence of intracoronary thrombus attached to the luminal surface with no detectable signs of fibrous cap rupture1,12. Thrombus was defined as a mass floating in or protruding into the lumen with a dimension of at least 250 µm. Quantitative OCT measurements included lesion length and lumen cross-sectional area (CSA) at lesion sites as well as reference sites. The inter-observer kappa coefficients for ML pattern, thrombus, and plaque rupture were 0.85, 0.86, and 0.89, respectively. The intra-observer kappa coefficients for ML phenotype, thrombus, and plaque rupture were 0.92, 0.95, and 0.95, respectively.
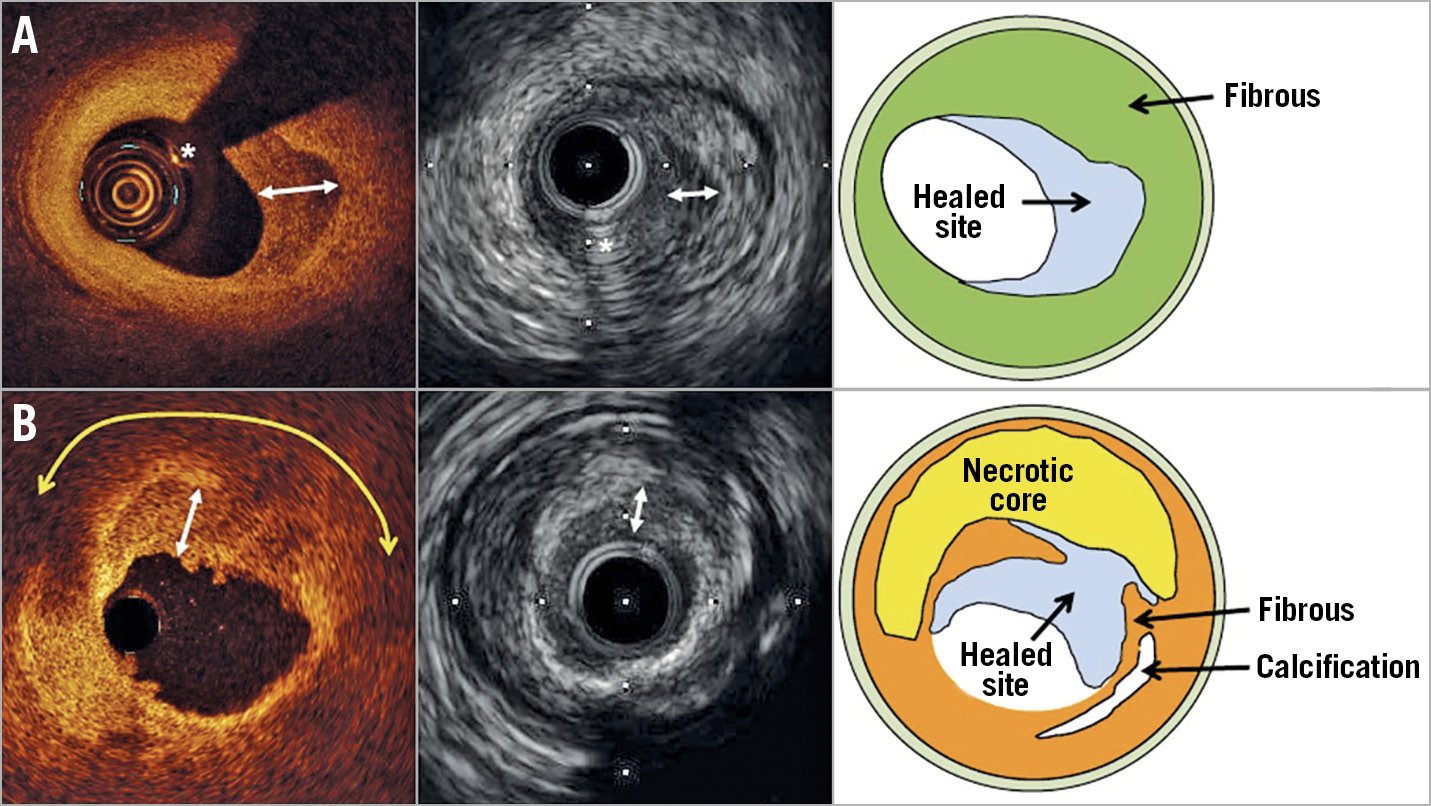
Figure 1. OCT and IVUS images of plaques with a multilayered pattern. OCT images showed multiple layers with distinct optical signals overlying a plaque without (A) or with (B) a large lipid core (yellow double arrow). Corresponding IVUS image shows multiple layers with distinct echogenicity. Two-headed arrows indicate the layer of healed lesion atop the original lesion. Asterisk indicates wire artefact.
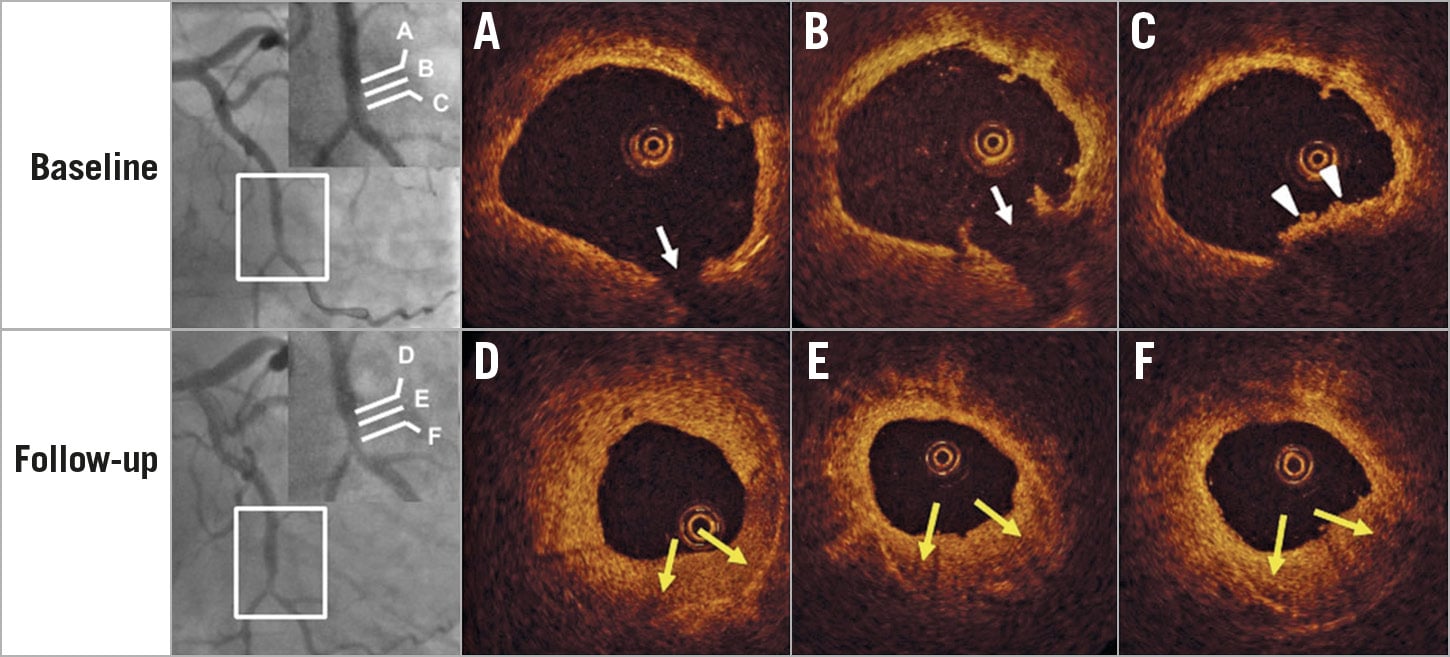
Figure 2. A case with silent plaque rupture in a non-culprit site healed with a multilayered pattern and luminal narrowing. Silent plaque rupture (arrows in A and B) with mural thrombus (arrowheads in C) was observed in the distal left circumflex. Six-month follow-up angiogram (bottom left) showed rapid progression at the site of previous rupture. OCT images demonstrated that previous rupture and thrombus healed with a multilayered pattern and significant lumen encroachment (yellow arrows in D, E, and F).
In a subgroup of patients, corresponding IVUS images were selected using fiduciary points such as calcifications, side branches, and stents. All IVUS images were analysed using semi-automatic software (echoPlaque; INDEC Systems, Mountain View, CA, USA). IVUS measurements included the external elastic membrane (EEM) CSA, lumen CSA, plaque plus media (P+M) CSA, and maximum and minimum atheroma diameter according to previously published methods.
QUANTITATIVE CORONARY ANGIOGRAPHY
Angiographic images were analysed using a quantitative coronary angiogram analysis program (CAAS 5.10.1; Pie Medical Imaging BV, Maastricht, the Netherlands). Reference diameter, minimum lumen diameter, lesion length, and percent diameter stenosis were measured.
CLINICAL OUTCOMES
Patients were followed up by telephone call or hospital visit at six months and 12 months. When a clinical event was identified, detailed data were collected at the study site and entered into the database. Clinical events included death from any cause, myocardial infarction, and target lesion revascularisation.
STATISTICAL ANALYSIS
Categorical data were compared using either a chi-square test or Fisher’s exact test, and are presented as counts (proportions). Continuous variables were compared using the independent samples t-test or Mann-Whitney test between the two groups. All statistical analyses were performed using SPSS, Version 18.0 (SPSS, Chicago, IL, USA). P-values <0.05 were considered statistically significant.
Results
BASELINE CHARACTERISTICS
Out of 204 patients, 119 (58.3%) showed an ML pattern in the culprit lesion. The baseline characteristics of patients with and without an ML pattern are summarised in Table 1. No significant difference was observed, except for a higher incidence of prior MI, use of angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker (ACEI/ARB), and presentation with SAP in patients with an ML pattern.
ANGIOGRAPHIC FINDINGS
Quantitative coronary angiography data are detailed in Table 2. Patients with the ML pattern had a smaller minimum lumen diameter (MLD), a higher degree of diameter stenosis, and greater lesion length. Complex lesions (type B2/C) were more frequent in patients with the ML pattern.
THE PREVALENCE OF THE ML PATTERN
Overall, the prevalence of the ML pattern was 58.3% in culprit sites. It was more frequently detected in patients with SAP compared to those with ACS (75.0% vs 51.4%, p=0.002), whereas there was no significant difference in the incidence of the ML pattern between patients with STEMI, NSTEMI, and unstable angina pectoris (UAP) (Figure 3A, Figure 3B). Patients with prior MI displayed a higher prevalence of the ML pattern compared to patients without prior MI (74.4% vs 54.5%, p=0.024) (Figure 3C).
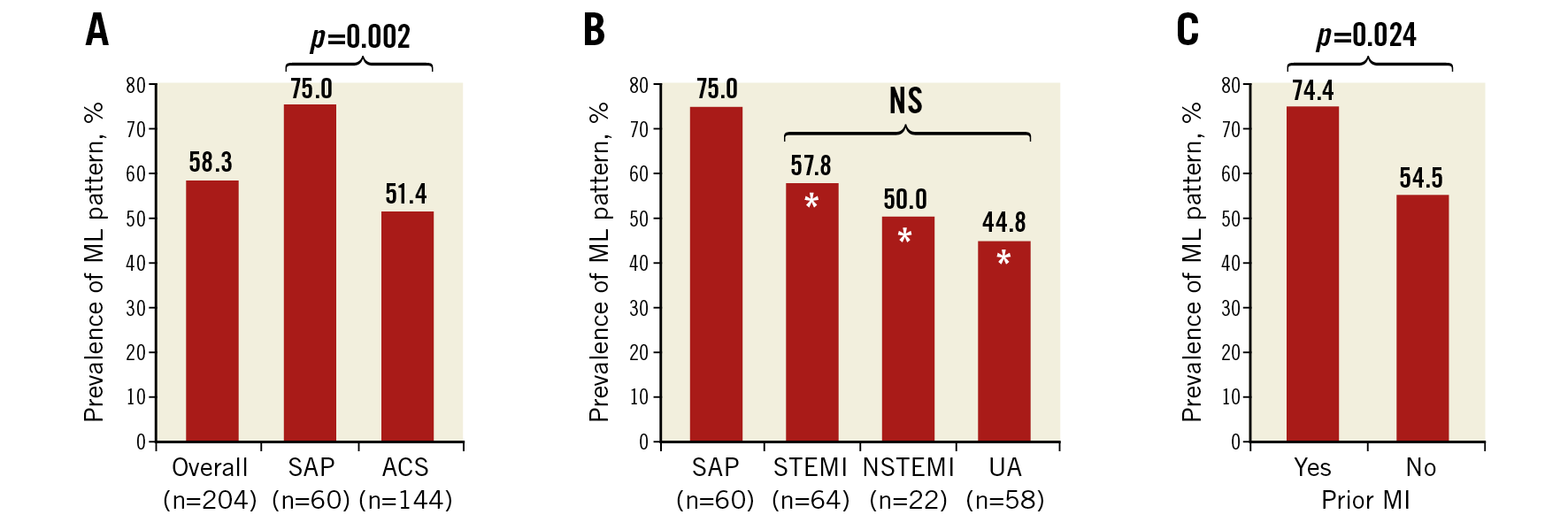
Figure 3. Prevalence of plaque with multilayered pattern according to clinical presentations. The overall prevalence of multilayered (ML) pattern was 58.3% in the whole cohort. ML pattern was more frequently observed in patients with SAP than in those with ACS (A), while no difference was observed between those with STEMI, NSTEMI, and UAP (B). Higher prevalence of ML pattern was observed in patients with prior MI compared to those without (C). *p<0.05 compared with SAP group.
ML PATTERN AND DEGREE OF STENOSIS
The ML pattern was more prevalent in patients with severe (≥75%) stenosis compared to those with less severe (<75%) stenosis (72% vs 39%, p<0.001). This difference was observed in both the ACS cohort (65% vs 30%, p<0.001) and the SAP cohort (91% vs 56%, p=0.002) (Figure 4A). The prevalence of the ML pattern increased with increased degrees of cross-sectional area stenosis (Figure 4B).
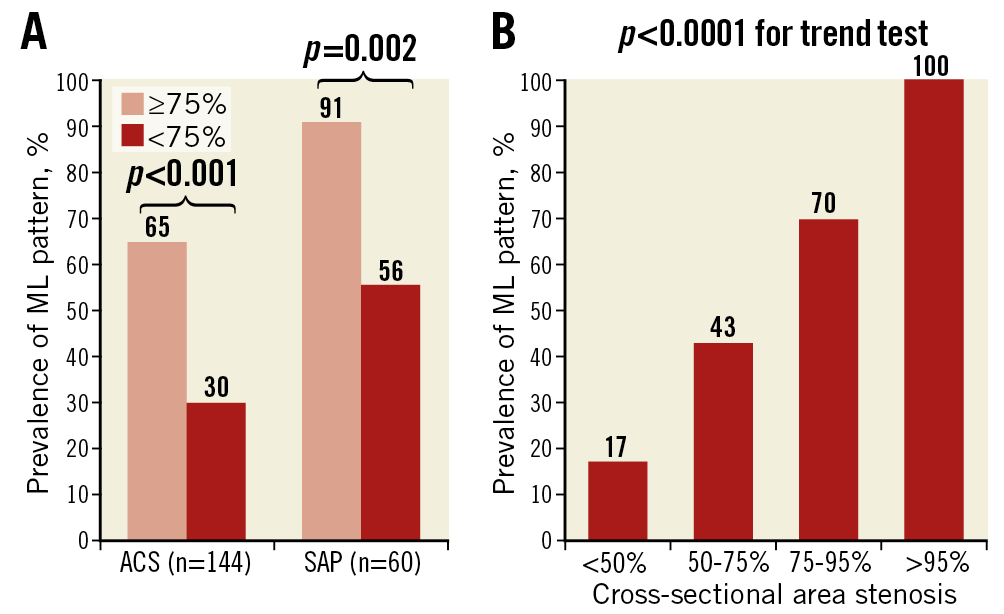
Figure 4. Prevalence of multilayered pattern according to the degree of stenosis. Higher prevalence of ML pattern was consistently observed in patients with critical stenosis compared to those without, regardless of clinical presentation (A). The prevalence of ML pattern increased with increased degrees of cross-sectional area stenosis (B) (p<0.0001, Cochran-Armitage trend test)
OCT FINDINGS
Qualitative and quantitative OCT data are presented in Table 3. The prevalence of lipid plaque, TCFA, plaque rupture, plaque erosion, and microchannels was similar between patients with and without the ML pattern (Table 3). Thrombus tends to be seen less frequently in patients with the ML pattern, probably due to more frequent presentation with SAP rather than ACS in this cohort. Quantitative OCT analysis showed that patients with the ML pattern had smaller minimum lumen area (MLA), greater CSA stenosis, and greater longitudinal length (Table 3, Figure 5).
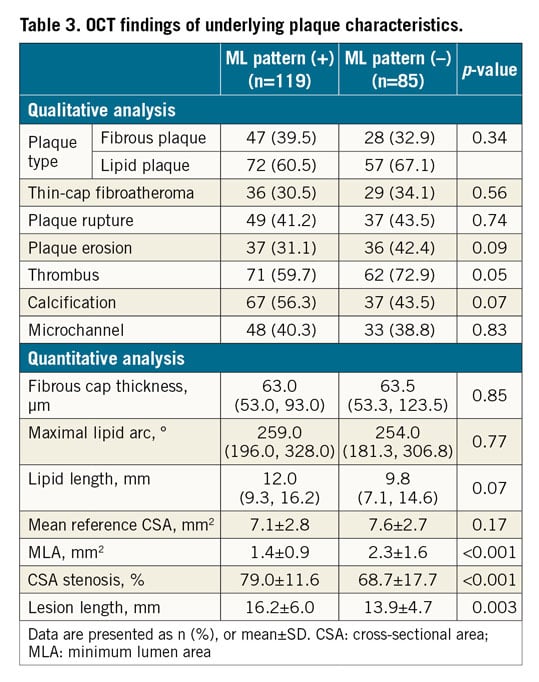
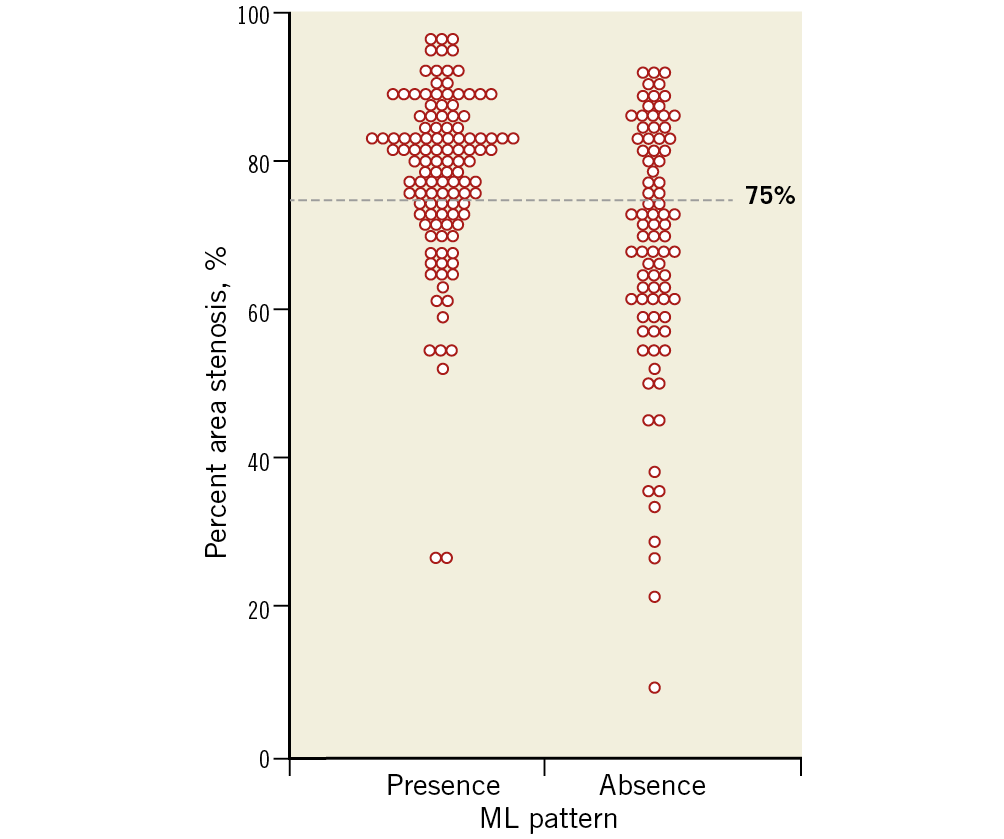
Figure 5. Distribution of percent area stenosis according to the presence of the multilayered pattern. Higher degree luminal stenosis (>75% area stenosis) was more frequently observed in lesions with the ML pattern.
IVUS FINDINGS
Forty-five patients (37 ACS and eight SAP) underwent both OCT and IVUS imaging of the culprit vessel. Within this subgroup, OCT identified 25 patients with the ML pattern and 20 without. IVUS findings in these 45 patients are summarised in Table 4. Plaque-related parameters at the lesion site were similar between the two groups, except for smaller plaque eccentricity in the ML pattern group. Negative remodelling was more frequently observed in the ML pattern group (56% vs 20%, p=0.018), whereas positive remodelling was more frequently seen in the non-ML pattern group (50% vs 20%, p=0.034) (Figure 6).
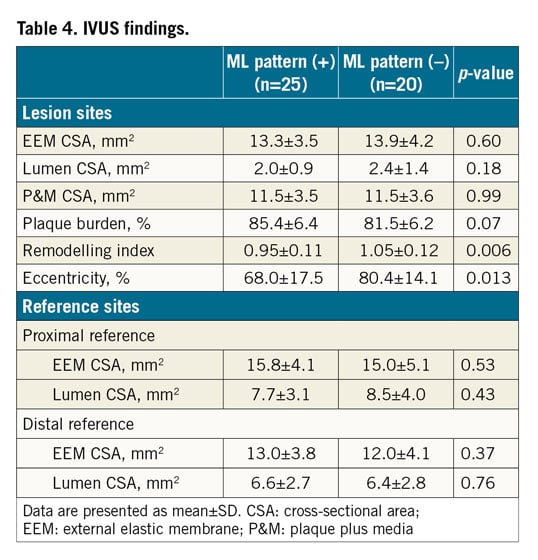
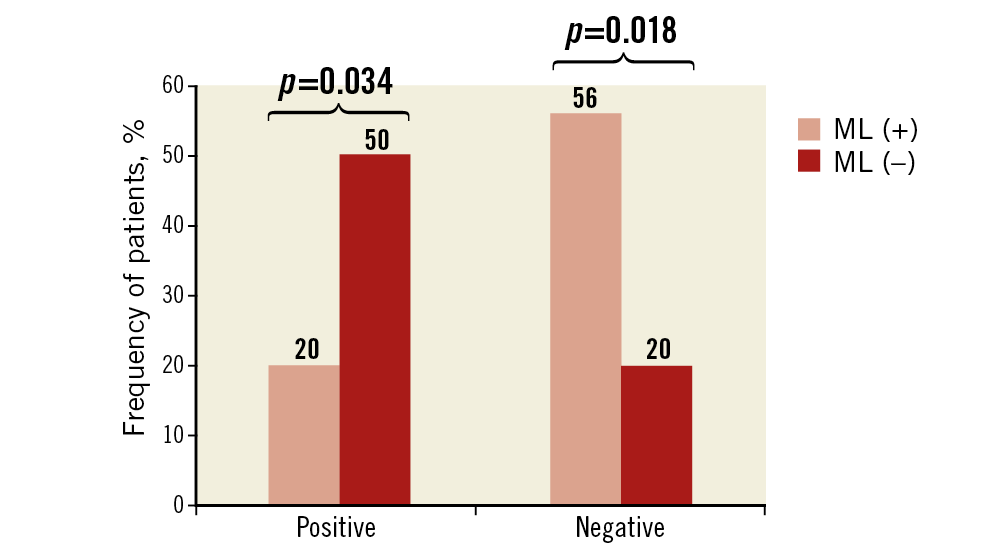
Figure 6. Remodelling in patients with and without the multilayered pattern in culprit lesions.
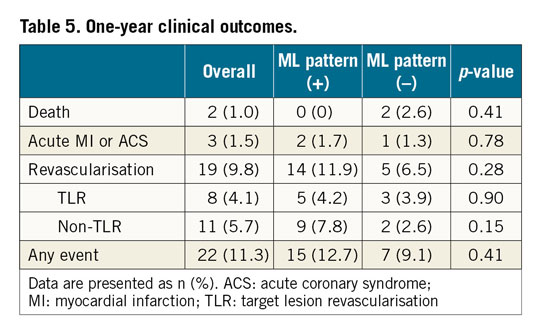
CLINICAL OUTCOMES AT ONE YEAR
One-year follow-up data were obtained in 193 (94.6%) patients. The incidence of major adverse cardiac events (MACE) at one-year follow-up is detailed in Table 5. The overall incidence of MACE was 11.3%. There was no significant difference in event rates between the two groups.
Discussion
To the best of our knowledge, this is the first in vivo study investigating the prevalence and clinical significance of plaque with an ML pattern. The main findings of the current study are: (1) the ML pattern was present in over half of the patients and was more frequently observed in patients with SAP compared to ACS; (2) patients with an ML pattern at the culprit site had a higher degree of luminal stenosis, longer and more complex lesions; and (3) an ML pattern was associated with negative remodelling on IVUS.
IN VIVO DETECTION OF HEALED PLAQUES BY INTRACORONARY OCT
The ML pattern is considered to represent healed sites of repeated silent plaque rupture or erosion2,13,14,15. Autopsy studies have shown that the sites of healed plaque rupture or erosion are characterised by multiple layers of distinct tissue. On OCT imaging, lesions in the early stage of healing appear as a layer of high OCT signal representing the original fibrous cap (type I collagen), covered by layers of low OCT signal representing loose newly-formed type III collagen2,6. The interface between these layers of tissue can be detected on imaging as a band of high signal backscatter, and several studies using different imaging modalities (IVUS and magnetic resonance imaging [MRI]) used this appearance to define healed lesions6,16,17. The resolution of both IVUS and MRI, however, is limited. OCT is a promising imaging modality for in vivo detection of this layered structure. Otsuka et al have recently compared the OCT appearance of healed plaque directly with pathology, and proposed that healed plaque can be detected by OCT using the landmark of multiple tissue layers6. A recent study demonstrated that OCT-derived healed coronary plaques showed high positive and negative predictive values for predicting histologically defined healed coronary plaques. Besides, 77% of the target lesion in patients with stable ischaemic heart disease have OCT-derived healed coronary plaques, which was consistent with our findings8.
PREVALENCE OF HEALED PLAQUE
Burke et al investigated 142 men who experienced sudden coronary death, and noted healed rupture in 61% of cases and an even greater prevalence in cases with stable plaque (80%)15. In the present study, the ML pattern characterising healed plaques on OCT imaging was observed in 58% of patients with symptomatic atherosclerotic disease, which is consistent with pathology studies (61%)15. Souteyrand et al studied plaque healing for the first time in a serial OCT study in ACS patients, showing the layer appearance mainly in cases with ACS due to erosion18. More than half of the patients with ACS showed the ML pattern at the culprit site, indicating that plaque instability and subclinical thrombosis commonly occur before the development of cardiac events. Our study identified a greater prevalence of the ML pattern in patients with a history of prior MI compared to those without. This finding seems logical as patients who had survived a prior MI most likely experienced a thrombotic event and it also confirms that an OCT-defined ML pattern is the morphological fingerprint of a previous plaque rupture or erosion.
HEALED PLAQUES AND HIGH-GRADE STENOTIC LESIONS
Atherosclerotic plaques develop and gradually narrow the arterial lumen to a critical degree with a consequent supply-demand imbalance19. This concept was widely accepted until serial angiographic studies in patients with chronic stable ischaemic heart disease showed that plaque progression in these patients was phasic rather than linear20,21,22,23. It is reported that episodic plaque growth is mediated by plaque rupture or erosion with subsequent thrombosis healing24. An autopsy study reported that the majority of high-grade lesions were caused by rapid plaque growth triggered by prior episodes of rupture. In our study, we found that patients with an ML pattern compared with those without had a higher degree stenosis and more complicated lesions. Surprisingly, SAP had the highest frequency of the ML pattern compared to the other population, suggesting that SAP patients may have experienced more episodes of thrombosis and healing before the lumen narrowed to a haemodynamically critical degree. In contrast, patients with ACS had a lower frequency of the ML pattern, suggesting fewer thrombosis-healing cycles. This discrepancy may be explained by the dynamic response following thrombosis in these two populations. Both systemic (more smoking, higher level of low-density lipoprotein [LDL] and high-sensitivity C-reactive protein [hs-CRP] in ACS) and local (more TCFA in ACS) thrombogenic factors may play a role in the thrombosis healing process. The lower incidence of positive plaque remodelling in the ML group is possibly due to the fact that there were fewer patients with ACS and more with SAP.
Limitations
Several limitations should be considered in this study. First, this was a retrospective study. Second, the presence of calcification and/or thrombus overlying a lesion may reduce the ability to detect the ML pattern using OCT. Patients with severe calcification or significant residual thrombus were therefore excluded from this study. Third, in cases of plaque rupture or erosion that occurred in the distant past, the ML pattern may no longer be recognisable. The prevalence of healed plaque may therefore be underestimated when identified using the ML pattern. Fourth, the number of patients in this study with stable disease was relatively small. Fifth, in selected cases, thrombus aspiration before OCT imaging might have modified plaque morphology by causing plaque rupture or intimal fissure. However, in the cases with massive thrombus or an occluded artery, thrombus aspiration is necessary for OCT image acquisition. Sixth, some plaque components such as streaks of inflammatory cells may lead to a layer aspect and mimic plaque healing, which may cause false positive layer detection.
Conclusions
The current study demonstrates that the ML pattern, representing repeated episodes of thrombosis, is more frequently observed in patients with stable coronary artery disease than in those with ACS. Presence of the ML pattern was associated with a higher degree of stenosis, longer lesions, and non-significant larger plaque burden. The ML pattern correlated with lesion progression but not features of plaque vulnerability.
|
Impact on daily practice The ML pattern, an OCT signature of prior thrombosis, can be used to facilitate a better understanding of the mechanisms driving plaque progression. In addition, the presence of new plaque with the ML pattern at follow-up can also be used to evaluate the pharmacological effect of lipid-lowering and antithrombotic therapy on plaque stabilisation. The ML pattern may therefore represent a potential tool in designing and evaluating preventive therapeutic strategies. |
Funding
This work was supported by a research grant from the National Natural Science Foundation of China, Harbin, China (81827806/B.Y., and 81330033/B.Y., 81722025/H.J., 81671763/H.J., 81701804/H.S.) and the National Key R&D Program of China (grant no. 2016YFC1301100 to B.Y.), Open Foundation of Key Laboratory of Myocardial Ischemia, Harbin Medical University, Chinese Ministry of Education (KF201410/C.W.), Harbin Medical University Innovation Fund Foundation Research Project (YJSCX2014-44HYD/C.W.).
Conflict of interest statement
The authors have no conflicts of interest to declare.

