Abstract
Background: The relationship between in-stent calcified nodules (IS-CN) and second-generation drug-eluting stent (G2-DES) stent thrombosis (ST) remains uncertain.
Aims: We aimed to evaluate the prevalence, clinical demographic and long-term clinical outcomes after G2-DES ST with IS-CN.
Methods: The prespecified substudy of the REAL-ST registry (a retrospective, multicentre registry of patients with definite ST after first- and G2-DES implantation) enrolled patients who experienced definite G2-DES ST and who underwent pre-intervention intravascular ultrasound imaging at index ST events.
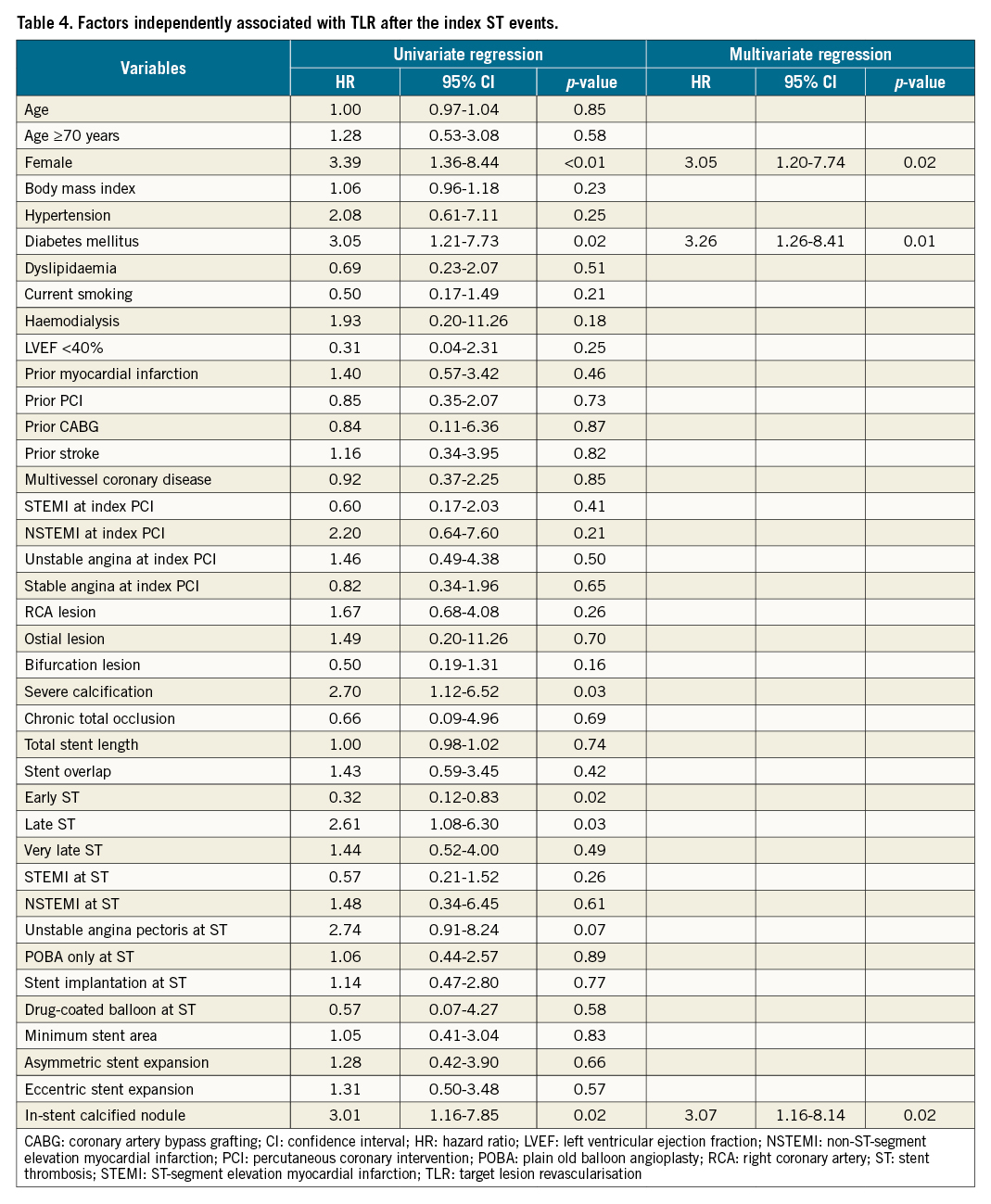
Results: IS-CN was observed in 15 out of 118 (13%) definite G2-DES ST cases. The multiple logistic regression model demonstrated that haemodialysis (odds ratio [OR] 12.27, 95% confidence interval [CI]: 1.56-94.54; p=0.02), proximal or mid-right coronary artery lesions (OR 12.79, 95% CI: 1.78-92.13; p=0.01) and severe calcification (OR 13.01, 95% CI: 1.18-142.94; p=0.04) were independently associated with ST with IS-CN. The cumulative 5-year incidence of target lesion revascularisation (TLR) after ST was significantly higher in the IS-CN group than in the non-IS-CN group (p=0.02). Independent predictors of TLR after the index ST events were female sex (hazard ratio [HR] 3.05, 95% CI: 1.20-7.74; p=0.02), diabetes mellitus (HR 3.26, 95% CI: 1.26-8.41; p=0.01) and IS-CN (HR 3.07, 95% CI: 1.16-8.14; p=0.02).
Conclusions: IS-CN may be one of the underlying mechanisms of G2-DES ST. Notably, IS-CN was associated with a higher TLR rate after the index ST events, suggesting the need for careful clinical follow-up of ST patients with IS-CN.
Introduction
Stent thrombosis (ST) is a serious complication following drug-eluting stent (DES) implantation1. Although the incidence of ST was significantly reduced as a new generation of DES have emerged, a recent Japanese nationwide REAL-ST registry (Retrospective Multicenter Registry of ST After First- and Second-Generation DES Implantation) demonstrated that the 1-year mortality after ST was equivalently high in patients treated with first-generation (G1-) DES and second-generation (G2-) DES2.
Several risk factors related to patient and lesion background for ST were identified in previous case-control studies. However, the mechanism of ST with G2-DES remains poorly understood. Recently, calcified nodules (CN) have become recognised as an important contributor to the occurrence of in-stent restenosis (ISR) and target lesion revascularisation (TLR) after DES implantation3. However, no previous study has evaluated the mechanistic contribution of CN progression to the occurrence of ST. Thus, we aimed to evaluate the prevalence, anatomic characteristics, clinical presentations and long-term clinical outcomes associated with the occurrence of G2-DES ST due to CN by evaluating the intravascular ultrasound (IVUS) data of the patients enrolled in the REAL-ST registry.
Methods
Study design
This study was a prespecified IVUS substudy of the REAL-ST registry (UMIN000025181), a retrospective, multicentre registry of patients with definite ST after G1- and G2-DES implantation at 46 Japanese percutaneous coronary intervention (PCI) institutions (Supplementary Appendix 1). The study design and main results are reported elsewhere4. Briefly, we retrospectively enrolled patients who fulfilled the following criteria: (1) patients who underwent PCI with DES between April 2004 and December 2016, or (2) those who had definite ST of DES between April 2004 and March 2017. A total of 655 patients with ST (G1-DES thrombosis, n=342; G2-DES thrombosis, n=313) were enrolled. Definite ST was defined according to the Academic Research Consortium criteria5.
The following patients were included in the IVUS substudy: 1) patients who experienced definite ST of G2-DES, and 2) those who had pre-intervention IVUS imaging at the time of definite ST. Patients with insufficient or poor quality IVUS imaging were excluded from the imaging substudy. In this study, the patients were divided into 2 groups according to the presence or absence of in-stent calcified nodules (IS-CN) on IVUS images at the time of definite ST. In the present study, we defined CN by the following IVUS features (a) protruding calcification (an echo signal brighter than the adventitia with acoustic shadowing) with its irregular surface and (b) the presence of calcification at adjacent proximal and distal segments according to the previously published manuscript6. An IS-CN was defined as a CN inside a stent (Figure 1), and if there was at least one IS-CN within the ST lesion, the patient was included in the IS-CN group. As a subgroup analysis, cases available for pre-IVUS imaging at index PCI were selected, and the IVUS images were analysed for the presence or absence of CN before stent implantation.
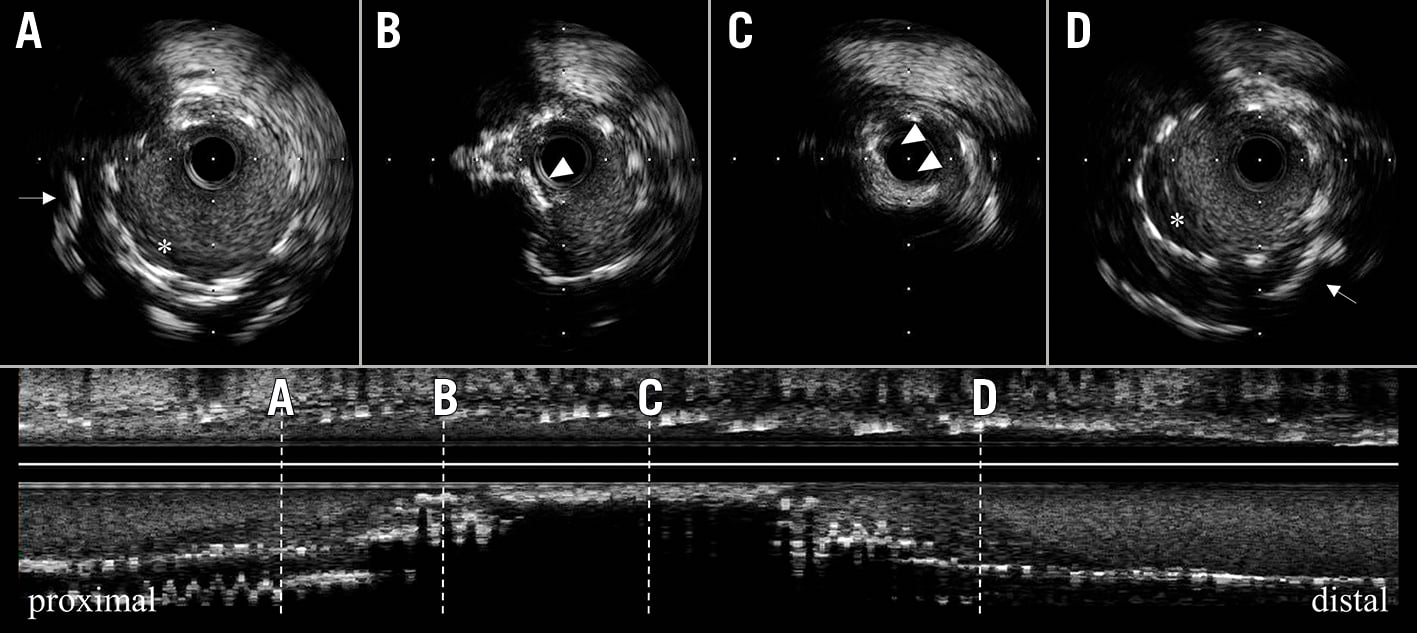
Figure 1. Representative case of an in-stent calcified nodule. Preprocedural IVUS imaging at late stent thrombosis. Cross-sections of IVUS shows A to D. Proximal (A) and distal (D) shows thrombus (asterisk) and deep calcification (white arrow). The mid-part (B and C) shows IS-CN (white arrow), it shows protruding calcification with an irregular surface. IS-CN: in-stent calcified nodule; IVUS: intravascular ultrasound
All IVUS images were analysed by two experienced investigators (Y. Takahashi and H. Otake), who were blinded to the angiographic data and clinical presentations. When there was discordance between the observers, a consensus reading was obtained from another investigator (T. Toba). Interobserver and intra-observer variability were assessed by the evaluation of all images by 2 independent observers and by the same observer at 2 separate time points.
The study protocol was approved by the ethics committees of all the participating centres. The study was conducted in accordance with the Declaration of Helsinki and its amendments. Written informed consent was waived due to the retrospective study design.
IVUS imaging and acquisition
IVUS procedures were performed in a standard fashion using an automated, motorised 0.5 or 1.0-mm/s pullback with commercially available imaging systems (Terumo, Boston Scientific or Phillips-Volcano). Inconsistent IVUS pullback was defined as IVUS images whose pullback speed was obviously inconsistent, in the sense that the images were stopping and suddenly restarting during pullback, thereby resulting in a >25% difference between the known target stent length and measured IVUS pullback length. Inconsistent pullback data were only used for qualitative analysis.
Angiography and IVUS analysis
Qualitative and quantitative coronary angiography analyses were performed independently by 2 experienced observers in an independent core laboratory (Kokura Memorial Hospital, Kitakyushu, Japan) blinded to the clinical information. Quantitative IVUS analysis was performed by an independent IVUS core laboratory (Kobe Cardiovascular Core Laboratory, Kobe, Japan) using validated planimetry software (EchoPlaque, Indec Systems) for quantitative and qualitative analyses. Details of IVUS measurement are described in Supplementary Appendix 2. The extent of stent expansion was evaluated using 3 parameters: % stent expansion, stent asymmetry index (AI), and stent eccentricity index (EI). The % stent expansion was calculated as the ratio of the minimum stent area (MSA) to the average reference lumen area7. The AI was calculated per lesion (1 - minimum stent diameter/maximum stent diameter throughout the entire stented segment)7. The minimum stent diameter was the minimal value of the stent diameter throughout the stented segment, and the maximum stent diameter was the maximal value of the maximal stent diameter throughout the stented segment. A lesion was deemed asymmetric if the AI value was more than 0.37. The stent EI was calculated as a parameter for the circularity of the cross-section, using the minimal stent diameter divided by the maximal stent diameter. The IVUS cross-sections with the lowest EI value per pullback were selected for analysis. A lesion with an EI of ≥0.7 was classified as concentric, while an EI of <0.7 was classified as eccentric7. Details of the qualitative IVUS analysis were described in Supplementary Appendix 3.
Clinical follow-up
The clinical follow-up data were obtained either from a review of the hospital records or by telephone contacts with the patients, relatives or referring physicians. The clinical follow-up data were used to evaluate the cumulative incidence of all-cause death, cardiac death, TLR and recurrent ST within 5 years following ST. Definite, recurrent ST was defined as the recurrence of acute coronary syndrome and angiographic evidence of thrombus in the same stent8. Patients who were lost to follow-up were censored on the last day with follow-up information. Follow-up intervals were calculated from the day of the index ST events.
Statistical analysis
Continuous variables with normal distributions are expressed as mean±standard deviation. Variables with non-normal distributions are expressed as median and interquartile ranges (25th-75th IQR). The Student’s t-test or analysis of variance was used to evaluate parametric continuous variables. The Mann-Whitney U test or the Kruskal-Wallis test was used for non-parametric variables. Categorical variables are expressed as frequencies with percentages. They were compared using the χ² or Fisher’s exact test. Multiple logistic regression analysis was used to assess the predictors of IS-CN in terms of patient and lesion characteristics.
In order to select variables for multivariate logistic regression analysis, the Least Absolute Shrinkage and Selection Operator (LASSO) analysis was performed on patient and lesion characteristics. The results are presented as odds ratios (ORs) with 95% confidence intervals (CIs). Incidence of all-cause death, cardiac death, TLR and recurrent ST after the index ST events were estimated using the Kaplan-Meier method. A log-rank test was used to analyse the significant differences among the groups. We estimated hazard ratios (HRs) of each outcome for IS-CN, and multivariate Cox regression analysis was used to adjust for baseline demographics4. The Cox proportional regression analysis was used to identify independent factors associated with TLR after index ST events. Baseline variables (p<0.05) in the univariate regression analysis were included in the multivariate logistic regression models. The results are presented as HRs with 95% CIs. All of the statistical analyses were performed using the SPSS for Windows version 25 (IBM) and SAS version 9.4 (SAS Institute).
Results
Patient flow and characteristics
Among the 313 patients who had definite ST of G2-DES, 121 patients fulfilled the inclusion criteria of the present IVUS substudy. Among them, 1 patient was excluded due to poor imaging quality and 2 due to incomplete data. Finally, a total of 118 patients with a total of 118 definite ST lesions that were subjected to IVUS-guided PCI for ST were enrolled. Based on the IVUS analysis at the time of ST, IS-CN was observed in 15 (13%) out of 118 definite ST cases of G2-DES. These patients were classified into the IS-CN group (n=15), while the rest were classified into the non-IS-CN group (n=103) (Supplementary Figure 1). Intra- and interobserver kappa for IS-CN were 0.96 and 0.88, respectively. Qualitative IVUS analysis was possible in 118 cases (IS-CN group, n=15; non-IS-CN group, n=103). After excluding cases with inconsistent IVUS pullback, volumetric IVUS analysis was available for the 93 cases (14 in the IS-CN group and 79 in the non-IS-CN group).
Patients with IS-CN had a higher prevalence of a history of haemodialysis, ostial lesions, severely calcified lesions and rotational atherectomy usage at the index PCI. Additionally, patients with IS-CN had a higher prevalence of right coronary artery (RCA) lesions (60% vs 23%), a lower prevalence of left main coronary artery and left anterior descending coronary artery lesions (27% vs 64%), and fewer bifurcation lesions than those without IS-CN (Table 1).
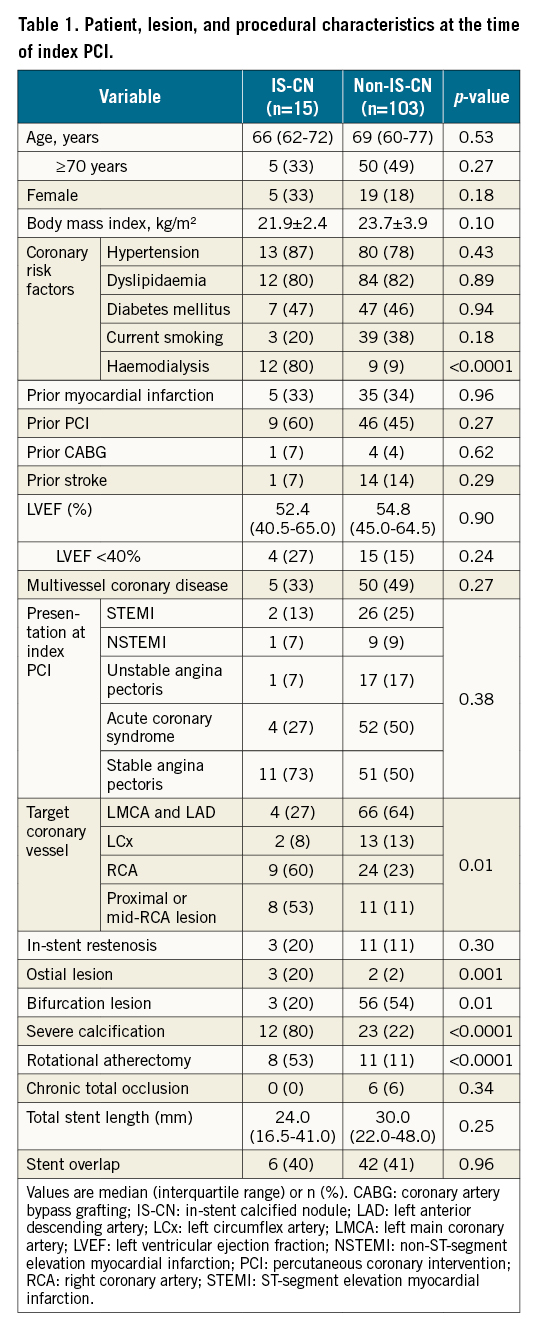
Table 2 shows the clinical presentation and treatment at the time of ST. Late ST was predominant in the IS-CN group (67%), while early ST was predominant in the non-IS-CN group (63%). The IS-CN group had a higher rate of thienopyridine monotherapy compared with the non-IS-CN group. The incidence of non-ST-segment elevation myocardial infarction occurred more often in the IS-CN group than in the non-IS-CN group (27% vs 6%). Multivariate analysis was performed using variables (age, body mass index, haemodialysis, proximal or mid-RCA lesions, severe calcification and total stent length) obtained from the LASSO analysis and demonstrated that haemodialysis (OR 12.27, 95% CI: 1.56-94.54; p=0.02), proximal or mid-RCA lesions (OR 12.79, 95% CI: 1.78-92.13; p=0.01) and severe calcification (OR 13.01, 95% CI: 1.18-142.94; p=0.04) were independently associated with IS-CN (Supplementary Table 1).
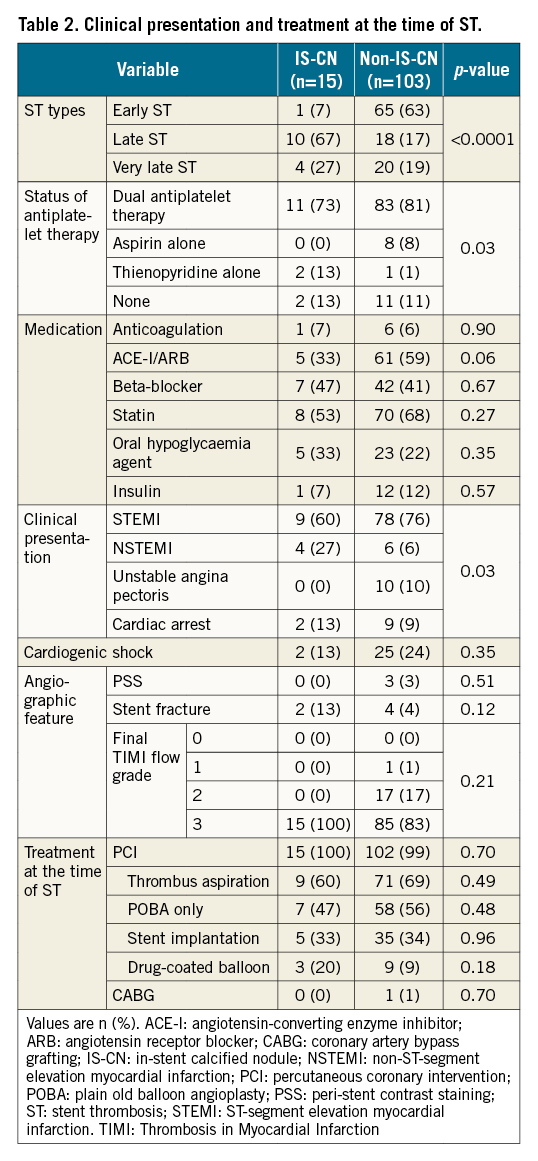
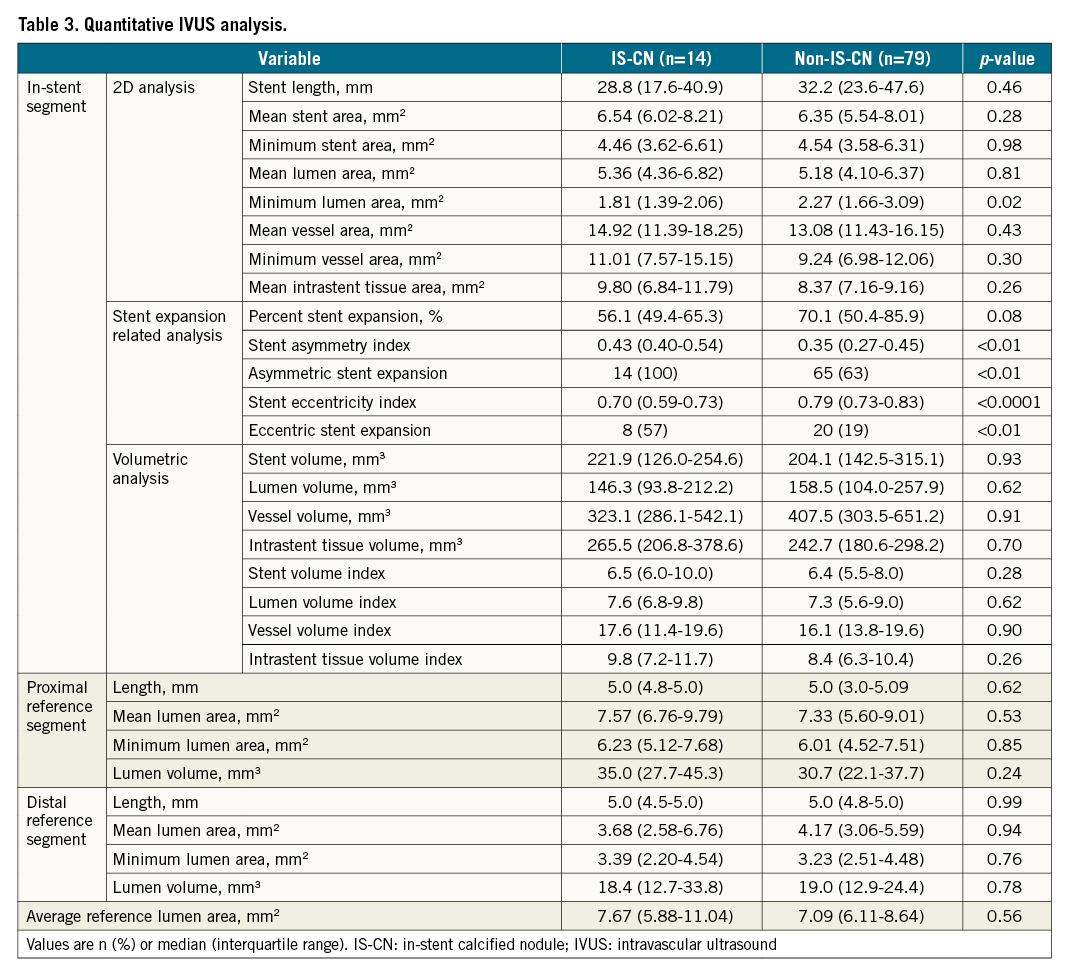
Qualitative and quantitative IVUS findings
The results of qualitative IVUS analysis at the time of ST are summarised in Supplementary Table 2. The incidence of severe calcified lesions under the stent was significantly higher in the IS-CN group compared with the non-IS-CN group (100% vs 36%; p<0.0001). Table 3 summarises the results of the quantitative IVUS analysis. Although the mean and minimum stent area were comparable, the minimum lumen area was significantly smaller in the IS-CN group than in the non-IS-CN group (Table 3). The percentage of stent expansion relative to the reference luminal area tended to be smaller in the IS-CN group compared with that in the non-IS-CN group. The stent AI was significantly larger in the IS-CN group than in the non-IS-CN group. The incidence of lesions with asymmetric stent expansion was 100% in the IS-CN group and 63% in the non-IS-CN group (p<0.01). The stent EI was significantly lower in the IS-CN group than in the non-IS-CN group. The incidence of lesions with eccentric stent expansion was significantly higher in the IS-CN group than in the non-IS-CN group (47% vs 19%; p<0.01) (Table 3).
Subgroup analysis of cases available for pre-IVUS imaging at index PCI
Among 118 definite ST cases of G2-DES (IS-CN: 15 patients; non-IS-CN: 103 patients), 46 patients were available for the preprocedural IVUS image analysis at index PCI (IS-CN: 9 patients; non-IS-CN: 37 patients). Among 9 patients with IS-CN ST, 8 (89%) had CN at index PCI before stenting, while only 5 out of 37 (14%) had CN in the non-IS-CN group. The presence of CN at initial PCI was significantly higher in the IS-CN group than in the non-IS-CN group (89% vs 14%; p<0.0001).
Clinical outcomes
The cumulative 5-year incidence of TLR after ST was significantly higher in the IS-CN group compared with that in the non-IS-CN group (62.7% vs 21.5%; HR 3.01, 95% CI: 1.16-7.85; p=0.02). The incidence of all-cause death, cardiac death, and recurrent ST was not statistically different between the 2 groups (Figure 2, Supplementary Table 3). A Cox proportional hazards model demonstrated that female sex (HR 3.05, 95% CI: 1.20-7.74; p=0.02), diabetes mellitus (HR 3.26, 95% CI: 1.26-8.41; p=0.01) and the presence of IS-CN on IVUS images at the time of ST (HR 3.07, 95% CI: 1.16-8.14; p=0.02) were independently associated with subsequent TLR after the index ST events (Table 4).
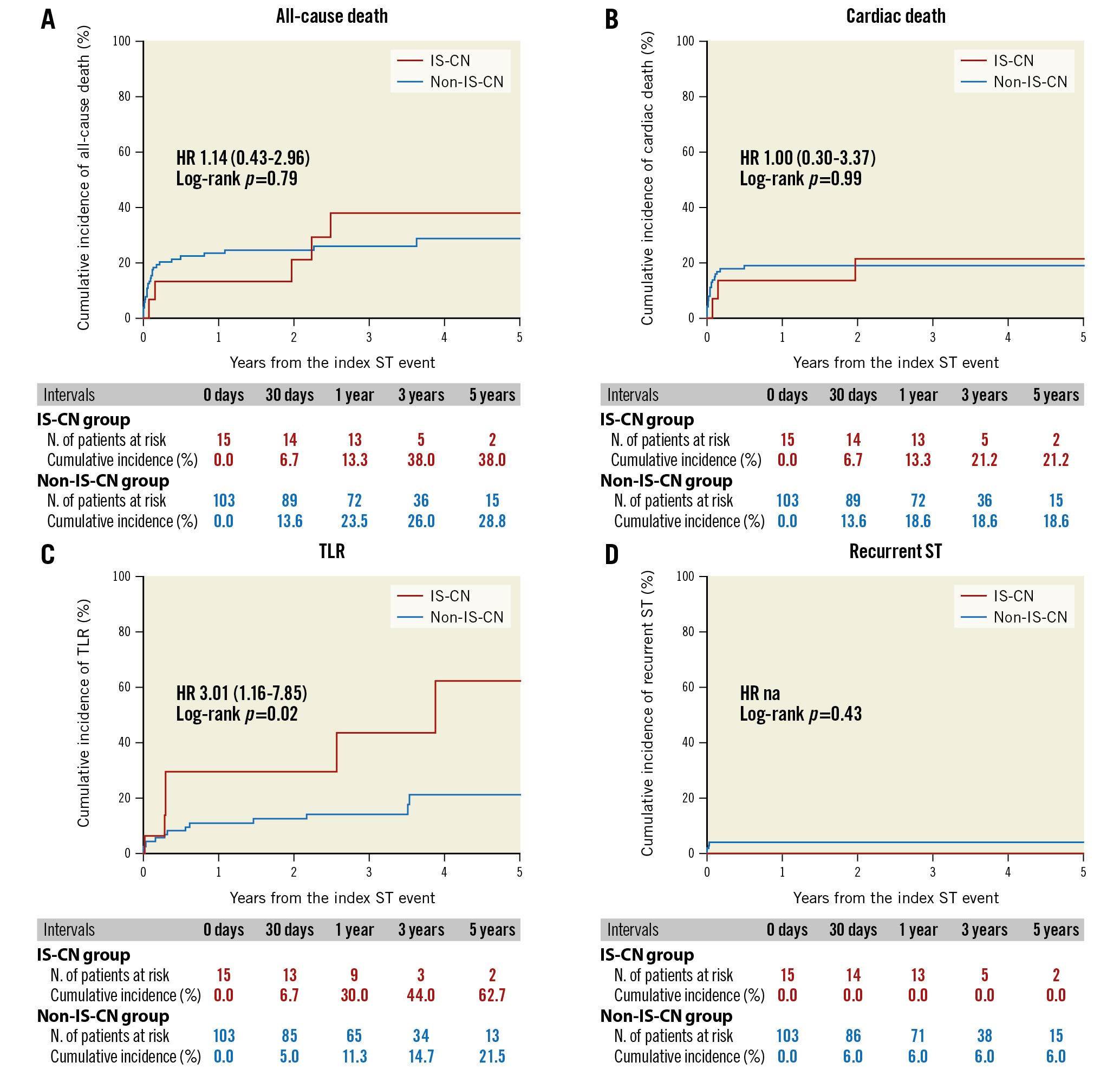
Figure 2. Clinical events after definite stent thrombosis. A) All-cause death, B) cardiac death, C) target lesion revascularisation and D) recurrent stent thrombosis. HR: hazard ratio; IS-CN: in-stent calcified nodule; na: not applicable; ST: stent thrombosis; TLR: target lesion revascularisation
Discussion
The main findings of the current study can be summarised as follows: (1) IS-CN was observed in 15 out of 118 (13%) definite ST cases of G2-DES; (2) haemodialysis and proximal or mid-RCA lesions were independently associated with ST with IS-CN; (3) stent expansion in the IS-CN group was more asymmetric and eccentric than those without IS-CN; and (4) the cases with ST with IS-CN were independently associated with a higher risk for TLR after the index ST events.
Several previous studies reported a potential relationship between the IS-CN and ISR. According to the previous studies, the prevalence of IS-CN in ISR lesions varied from 5% to 31%391011. While many studies reported the relationship between IS-CN and ISR, only a few case reports reported its relationship with ST. Mori et al reported 2 cases wherein CN resulted in thrombus formation inside previously implanted stents12. However, these patients died for non-cardiac reasons, and ST due to CN was accidentally found as a chronic total occlusion of previously implanted stents. Thus, the current study is the first to clarify the prevalence of IS-CN among a series of patients who experienced acute onset of definite ST of G2-DES.
In the current study, we found that haemodialysis was associated with ST lesions with IS-CN. End-stage renal disease and haemodialysis were reported as independent risk factors for CN in the native coronary artery13. The association between haemodialysis and IS-CN was also demonstrated. In a recent pathological study of ISR tissues obtained through directional coronary atherectomy, Nakamura et al demonstrated that IS-CN was the major histological characteristic causing ISR in patients undergoing haemodialysis10. These findings indicate that haemodialysis is a common risk factor for the development of CN, regardless of whether it is in the de novo lesion or the in-stent segment. Additionally, our study indicates that haemodialysis can be a common risk factor not only for ISR but also for ST with IS-CN.
CN has its own predominant site in the human coronary artery. Lee et al demonstrated that in a series of native coronary artery diseases CN was located more frequently in the ostial or mid-RCA13. In addition to the native coronary artery, Nakamura et al reported that 57% (4 of 7 lesions) of IS-CN were located in the RCA10. In the present study, 60% of ST with CN was observed in RCA lesions, and proximal and mid-RCA lesions were independently associated with ST due to IS-CN. Although the mechanism of IS-CN remains poorly understood, the association between coronary hinge motion, severe calcified lesions and the occurrence of CN has been frequently reported. Interestingly, there was a numerically higher incidence of stent fractures in ST lesions with IS-CN than in those without (13% vs 4%; p=0.12), although the difference was not statistically significant due to the limited sample size. Thus, the presence of hinge motion might affect the progression of CN, even in the stented segment. In contrast, a recent IVUS study by Sugane et al reported that 82.4% of ISR in CN lesions was driven by CN regrowth at the same location where CNs were originally observed before stenting3. Albeit the subgroup analysis was with a limited sample size, our results showed that 89% of IS-CN lesions have CN at index PCI, in line with the previous study3. Thus, we speculate that the presence of CN under the stent and regrowth after stenting could be another potential mechanism of IS-CN leading to ST (Central illustration).
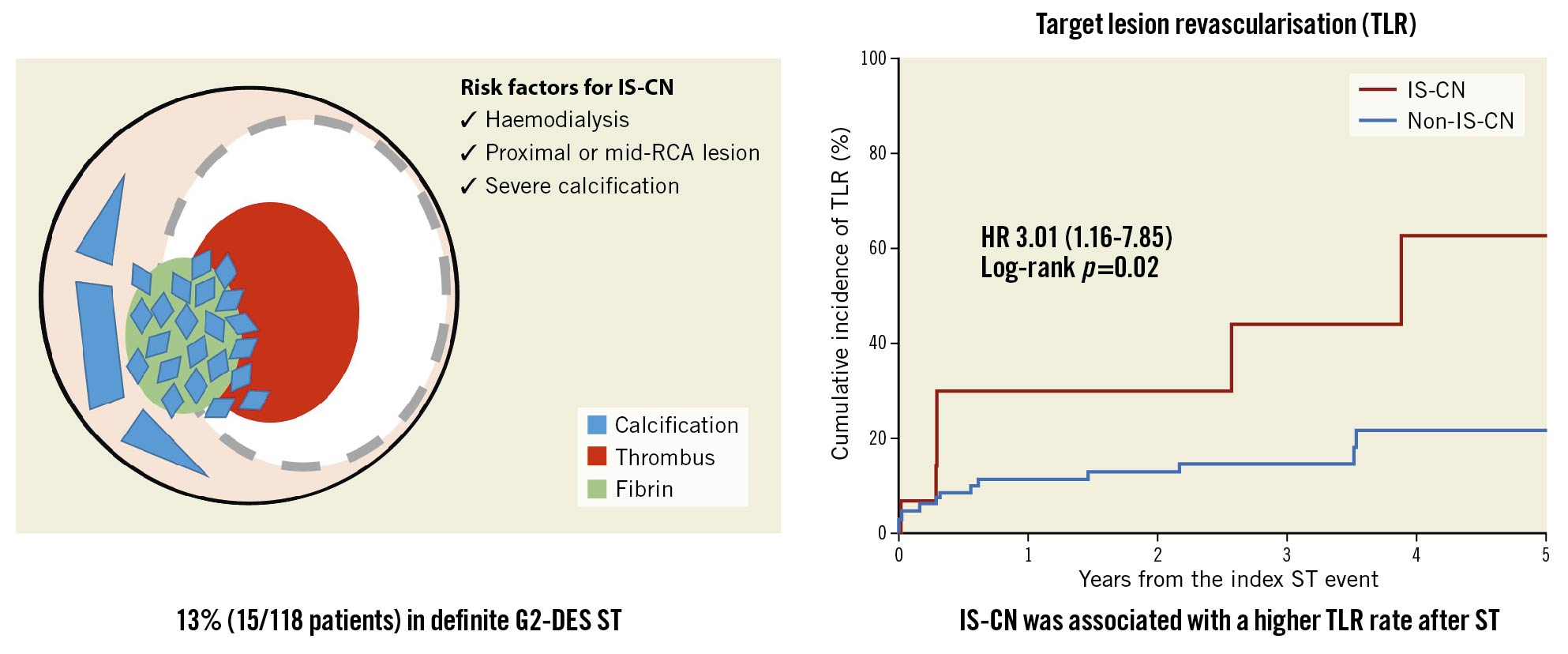
Central illustration. Schema of prevalence and risk factors for stent thrombosis with in-stent calcified nodules and outcome of in-stent calcified nodules in stent thrombosis lesion. G2-DES: second-generation drug-eluting stent; HR: hazard ratio; IS-CN: in-stent calcified nodule; IVUS: intravascular ultrasound; RCA: right coronary artery; ST: stent thrombosis; TLR: target lesion revascularisation
Previous reports consistently reported that minimum stent area and residual stenosis were important contributors to the occurrence of ST1415. However, we did not observe any statistical differences between the 2 groups in these variables, since all the cases enrolled in this substudy were ST cases. Instead, the IS-CN group had a significantly larger stent AI and lower stent EI compared with the non-IS-CN group, suggesting that the IS-CN group had more asymmetric and eccentric stent expansion than the non-IS-CN group. Based on these findings, we consider that the presence of CN in the native coronary artery may induce asymmetric and eccentric stent expansion, which may progress IS-CN development through accelerated fibrin and thrombus formation on top of the protruding CN inside the stented segment, leading to the occurrence of ST. Importantly, the present study showed that the presence of IS-CN in ST lesions was independently associated with TLR after the index ST events. The presence of IS-CN and more asymmetric and eccentric stent expansion might induce unfavourable clinical outcomes after the treatment of ST. Given these findings, the careful clinical follow-up should be considered for patients with IS-CN at the time of the index ST events.
Limitations
There are several limitations of the present study. First, among patients with definite G2-DES ST, we retrospectively enrolled those who had pre-intervention IVUS imaging at the time of ST. Thus, selection bias might have existed. Second, a relatively large number of cases were excluded for IVUS analysis, mainly due to the lack of IVUS images available for the analysis. Also, a relatively large number of cases (25 patients from 118 patients) were excluded due to inconsistent pullback for quantitative IVUS analysis. However, this lesion selection reinforced the reliability of our findings because of the elimination of cases that could hamper accurate quantitative evaluation. Also, post-PCI IVUS images were not analysed. Third, since the IVUS imaging at index PCI was relatively small, the association between index PCI and ST could not be adequately assessed in the present study. Fourth, we could not assess the prevalence of IS-CN in G1-DES ST cases due to the lack of IVUS data collected in the core laboratory, although comparing the prevalence of IS-CN between G1- and G2-DES ST cases might add new insights into the underlying mechanism of DES-ST. Fifth, CN was diagnosed according to its definition on IVUS but not optical coherence tomography (OCT), which may overdiagnose CN. A further study with OCT might be needed to confirm our findings. Finally, this study has no statistical power for clinical endpoints. Although the difference for TLR between groups is highly plausible, this result still needs to be considered hypothesis-generating. Due to the small sample size, LASSO analysis did not identify variables associated with TLR. Also, due to the small sample size, the proportional hazards assumption may not be correct for death and cardiac death. However, there was statistically no significant difference between the 2 groups. A further study with a larger sample size might be necessary to confirm our findings.
Conclusions
The IVUS substudy of the REAL-ST registry suggested that IS-CN might be one of the underlying mechanisms of definite ST after G2-DES implantation. Given that definite ST patients with IS-CN were associated with a higher TLR rate after the index ST events than those without, careful clinical follow-up should be considered for these patients.
Impact on daily practice
In the REAL-ST registry, IS-CN was observed in 13% of definite G2-DES ST cases. IS-CN might be one of the underlying mechanisms of definite ST after G2-DES implantation. Definite ST patients with IS-CN were associated with higher TLR rates after ST than those without, suggesting the need for careful clinical follow-up of these patients.
Acknowledgements
We appreciate the efforts of the investigators in the 46 participating centres.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

