Abstract
About one-third of patients undergoing transcatheter aortic valve implantation (TAVI) use oral anticoagulants (OAC), mainly due to atrial fibrillation. General guidelines advise interrupting OAC in patients with a high risk of bleeding undergoing interventions. However, preliminary observational data suggest that the continuation of OAC during TAVI is safe and may reduce the risk of periprocedural thromboembolic events. The Periprocedural Continuation Versus Interruption of Oral Anticoagulant Drugs During Transcatheter Aortic Valve Implantation (POPular PAUSE TAVI) is a multicentre, randomised clinical trial with open-label treatment and blinded endpoint assessment. Patients are randomised 1:1 to periprocedural continuation versus interruption of OAC and are stratified for vitamin K antagonist or direct oral anticoagulant use. The primary endpoint is a composite of cardiovascular mortality, all stroke, myocardial infarction, major vascular complications and type 2-4 bleeding within 30 days after TAVI, according to the Valve Academic Research Consortium-3 criteria. Secondary endpoints include separate individual and composite outcomes, quality of life and cost-effectiveness. Since continuation of OAC is associated with the ancillary benefit that it simplifies periprocedural management, the primary outcome is first analysed for non-inferiority; if non-inferiority is proven, superiority will be tested. Recruitment started in November 2020, and the trial will continue until a total of 858 patients have been included and followed for 90 days. In summary, POPular PAUSE TAVI is the first randomised clinical trial to assess the safety and efficacy of periprocedural continuation versus interruption of OAC in patients undergoing TAVI.
Introduction
Transcatheter aortic valve implantation (TAVI) is an appropriate therapeutic option for elderly patients with symptomatic severe aortic stenosis or for younger patients at increased surgical risk12. Despite considerable technical advances over the years, both stroke and bleeding remain feared complications, which negatively impact recovery and survival after TAVI345. About 35% of patients undergoing TAVI have an indication for oral anticoagulation (OAC), mainly due to concomitant atrial fibrillation (AF)67. These patients represent a high-risk subset because they are generally older and have more comorbidities, as well as increased associated frailty78. In addition, the use of OAC in these patients is inherently related to both their bleeding and thromboembolic risks. These risks are especially relevant in the periprocedural period, when interruption of OAC during TAVI may increase the risk of thromboembolism, whilst continuation may increase the risk of bleeding. Guidelines advise interrupting OAC in patients at high risk of bleeding undergoing interventions, but the optimal strategy for patients undergoing TAVI is unknown910. Recently, a number of observational studies reported that the continuation of OAC during TAVI did not result in an increase in bleeding or vascular events11121314. Moreover, a signal towards a lower stroke risk was observed with continued OAC1113. Numerous limitations surround these analyses, which hamper implementation into clinical practice15. High-quality evidence regarding the optimal periprocedural OAC strategy is needed. Therefore, we designed the Periprocedural Continuation Versus Interruption of Oral Anticoagulant Drugs During Transcatheter Aortic Valve Implantation (POPular PAUSE TAVI) trial, which aims to assess the safety and efficacy of periprocedural continuation versus interruption of OAC in patients undergoing TAVI.
Methods
Study design
POPular PAUSE TAVI (ClinicalTrials.gov: NCT04437303) is a pragmatic, multicentre, randomised clinical trial which tests the hypothesis that periprocedural continuation of OAC is safe and might decrease thromboembolic events without an increase in bleeding complications at 30 days after TAVI16. A total of 858 patients will be randomised 1:1 to continuation versus interruption of OAC (Figure 1). The study was designed as a non-inferiority trial because, in addition to a potential reduction in thromboembolic events, continuation of OAC is associated with the ancillary benefit that it simplifies periprocedural management for both patients and staff. If non-inferiority is proven, superiority will be tested.
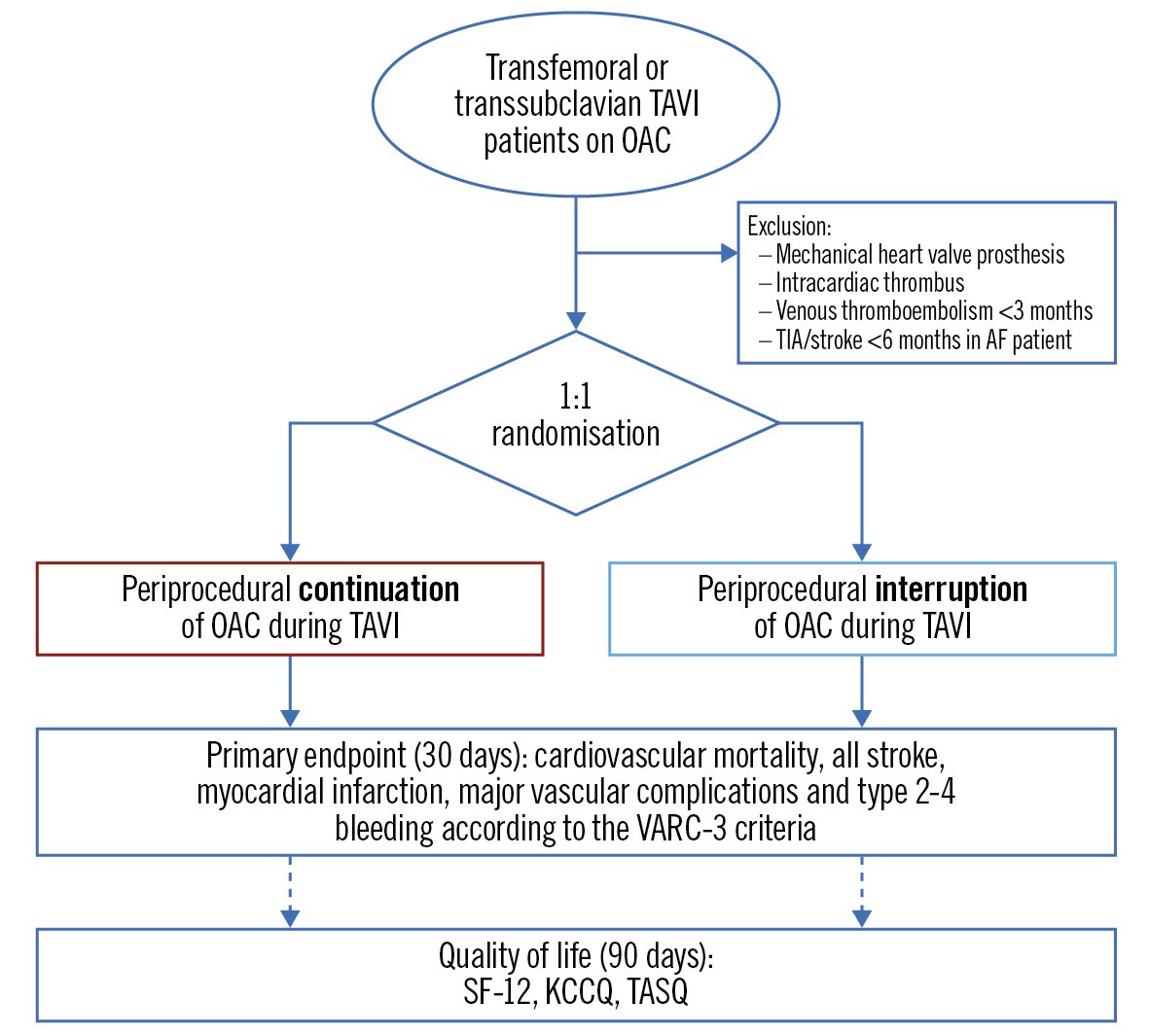
Figure 1. Flowchart for the POPular PAUSE TAVI trial. AF: atrial fibrillation; KCCQ: Kansas City Cardiomyopathy Questionnaire; OAC: oral anticoagulation; SF-12: Short Form-12; TASQ: Toronto Aortic Stenosis Questionnaire. TAVI: transcatheter aortic valve implantation; TIA: transient ischaemic attack; VARC: Valve Academic Research Consortium
Study population
Patients using OAC who are undergoing transfemoral or transsubclavian TAVI and who provide written informed consent are potentially eligible to participate in the study. The exclusion criteria apply to patients at high risk for thromboembolism for whom a true interruption of OAC (=interruption without bridging) is not an option, i.e., presence of a mechanical heart valve prosthesis, intracardiac thrombus, venous thromboembolism within 3 months before TAVI, or transient ischaemic attack (TIA) or stroke in patients with AF within 6 months before TAVI. To maximise the generalisability and to establish a “real-life” study population, all participating sites are strongly recommended to include patients on a consecutive basis. The study started in November 2020 and will continue until a total of 858 patients have been included and followed for 90 days. Currently, patients are being recruited at 21 study sites in the Netherlands, Belgium, Luxembourg, Denmark, Ireland and Italy. As of May 2023, a total of 625 patients have been successfully enrolled. Enrolment is expected to be complete in spring 2024.
Study procedures and treatment
After screening and enrolment, patients are randomised approximately 1-2 weeks before TAVI, to be able to inform them about the randomised strategy. Randomisation is performed within the REDCap eCRF randomisation module (Vanderbilt University). Patients are randomised in a 1:1 ratio, with variable block sizes stratified for the type of OAC (vitamin K antagonist [VKA] versus direct oral anticoagulant [DOAC]) and study site.
For the interruption group, a previously described strategy for patients undergoing a high bleeding risk procedure was adopted, as this was the standard of care for DOAC patients at the leading study site9. DOAC users interrupt their OAC 48 hours before TAVI, except for dabigatran users with renal insufficiency. Patients using dabigatran with an estimated glomerular filtration rate (eGFR) between 50 and 80 interrupt medication 72 hours before, and for those with an eGFR between 30 and 50, 96 hours before TAVI. VKA users interrupt acenocoumarol 72 hours before TAVI and phenprocoumon or warfarin 120 hours before TAVI. Bridging with low-molecular-weight heparin (LMWH) is not applied because patients at high thromboembolic risk are excluded (see above), and current evidence mainly shows an effect of increased perioperative bleeding without antithrombotic benefit in patients undergoing elective operations or invasive procedures17. Patients restart OAC after TAVI, as soon as it is deemed safe by the operator and/or treating physician − generally, in the evening or the next morning after TAVI.
Patients randomised to the continuation group continue their OAC even on the day of TAVI. VKA patients are dosed based on their usual target international normalised ratio (INR). When an evident clinical contraindication arises, e.g., ongoing bleeding, OAC may be interrupted upon discretion of the treating physician, as this is primarily a strategy study.
The TAVI procedures are performed according to the local protocols of each participating study site, including choice of valve type, cerebral embolic protection, per procedural heparin and protamine administration, and vascular closure, regardless of the randomised strategy. Follow-up visits to assess clinical outcomes are performed at discharge and 30 days after TAVI, which may be performed onsite or by telephone.
Study endpoints
The primary endpoint is a composite of cardiovascular mortality, all stroke, myocardial infarction, major vascular complications and type 2-4 bleeding within 30 days after TAVI, according to the Valve Academic Research Consortium (VARC)-3 criteria18. Secondary endpoints include separate components of the primary endpoint, as well as other composite endpoints (e.g., VARC-3 clinical safety and efficacy) at discharge and 30 days after TAVI.
Quality of life is assessed using the generic Short Form-12 questionnaire, the disease-specific Kansas City Cardiomyopathy Questionnaire19 and Toronto Aortic Stenosis Quality of Life Questionnaire (TASQ)20. These questionnaires are completed at baseline, and at 30 and 90 days after TAVI. TASQ was developed recently, specifically for patients with aortic stenosis, and consists of 5 domains: physical symptoms, physical limitations, emotional impact, social limitations and health expectations20. Other endpoints include New York Heart Association (NYHA) Class, hospital readmission and cost-effectiveness of the continued as compared to the interrupted OAC strategy, which will take into account the incidence of the study outcomes, impact on quality of life and related costs.
Funding and ethical considerations
This investigator-initiated trial is funded by the St. Antonius Research Fund and by the Netherlands Organization for Health Research and Development. There is no industry involvement in the trial. The study is conducted according to the principles of the Declaration of Helsinki, amended by the 64th WMA General Assembly, Fortaleza, Brazil, October 2013, and in accordance with the Medical Research Involving Human Subjects Act (WMO) and other guidelines, regulations, and acts. Ethics committees in each country and institutional review boards at each participating site authorised the clinical trial protocol. Monitoring is performed according to Good Clinical Practice (GCP) guidelines under the direction of the Research and Development Academy of St. Antonius Hospital. An independent Data Safety Monitoring Board (DSMB) was established to perform safety surveillance on the accruing study data to safeguard the interests of the trial participants. The board discusses the study protocol and (interim) outcomes and provides recommendations to the investigators on the further conduction of the trial. If the interim results are likely to convince a broad range of clinicians that one trial arm is clearly indicated or contraindicated, the DSMB will provide advice on whether recruitment should be terminated. A blinded clinical endpoint committee adjudicates all potential primary endpoints prior to presentation of the data to the DSMB. In addition, a yearly safety report is submitted to the accredited medical research ethics committees of the concerned member states.
Biochemical substudy
To facilitate interpretation of the clinical outcomes and gain further insight into the underlying pathophysiology of thromboembolic and bleeding events early after TAVI, a dedicated biochemical substudy was established, within the context of the main study. Blood samples are taken at baseline (T=1), 10 minutes after vascular closure (T=2), during the evening round on the day of TAVI (T=3), during the morning round on the first day after TAVI (T=4), during the morning round on the second day after TAVI (T=5), and at 6-week ambulatory follow-up (T=6). Multiple indicators of coagulation activation (e.g., thrombin generation, coagulation factors, prothrombin fragment F1+2, tissue factor, fibrinogen, thrombin-antithrombin complex) and platelet activation are measured and compared between the 2 study groups. Also, different types of von Willebrand Factor (vWF) and ADAMTS13 levels are measured to study their association with the severity of aortic stenosis. In addition, the effect of TAVI on the restoration of vWF and ADAMTS13 is evaluated, including their role as early markers of paravalvular leakage.
Sample size calculation
We anticipate an incidence of the primary composite endpoint of 17.5% in the interrupted OAC group and 13.5% in the continued OAC group. This is based on the event rates in cohort B of the POPular TAVI trial8 and on previous reports evaluating continuation versus interruption of OAC11. To provide the study with 90% power to demonstrate non-inferiority of the primary endpoint at a 1-sided alpha level of 2.5% and a non-inferiority margin of 4%, the sample size was calculated to be 858 patients, based on the formula proposed by Blackwelder21.
Statistical analysis
The primary analysis is a modified intention-to-treat (mITT) analysis. ITT is determined by randomisation; the modified aspect is the period during which there is a risk of developing any of the outcome events, which is defined as 5 days before until 30 days after TAVI. This was chosen because any event which occurs between the moment of randomisation and 5 days before TAVI is, by definition, not related to the study regimen. This period between randomisation and effectuating the study regimen is needed to be able to inform patients about the study regimen to be followed. Excluding the randomised patients who never received the intervention or control treatment from the primary analysis does not introduce bias and leads to a more informative analysis22, especially since this is a non-inferiority trial23, provided that the blinded adjudication committee makes this determination of exclusion.
The primary endpoint will be tested for non-inferiority to evaluate the safety of continuation of OAC compared to interruption of OAC. The absolute difference in the occurrence of the primary endpoint between these groups will be calculated with its 95% confidence interval (CI) and compared to the prespecified, absolute non-inferiority margin (M) of 0.04. This margin is based on clinical acceptability, because there are no data available to reliably establish a non-inferiority margin based on the preservation of a predetermined fraction of the efficacy of the control group compared with placebo, as recommended24. Equivalently, non-inferiority will be assessed using the formal test proposed by Blackwelder to assess hypotheses with a specified difference. Following the standard non-inferiority testing methodology, this 1-sided test will be evaluated at an alpha of 0.025. If non-inferiority is proven, superiority will be tested to evaluate the efficacy of continued compared to interrupted OAC. There will be no adjustment for type I error level for the final analyses, since this closed-testing procedure ensures that the overall experiment-wise error rate is maintained at the correct level when testing more than one hypothesis25. Besides the risk difference, the relative risk between both groups will also be calculated.
Discussion
POPular PAUSE TAVI is the first randomised clinical trial to assess the safety and efficacy of periprocedural continuation versus interruption of OAC in patients undergoing TAVI. Due to the lack of high-quality evidence, periprocedural OAC management varies considerably between TAVI centres26. Some centres interrupt OAC more than 1 week prior to TAVI, whilst others continue OAC throughout the periprocedural period. The applied strategy also differs depending on the use of VKA (more often continuation) or DOAC (more often interruption). This may be related to the rapid and predictable mechanism of action of DOACs, which makes interruption relatively easy as compared to VKA. Furthermore, of the centres that interrupt OAC, some use LMWH or antiplatelet therapy for “bridging”, whereas others do not.
Guidelines advise interrupting OAC in patients with a high risk of bleeding undergoing interventions910. However, there is increasing evidence that for specific cardiac procedures continuation of OAC is at least as safe and effective as interruption. In the BRUISE CONTROL trial, a strategy of continued warfarin at the time of pacemaker or implantable cardioverter-defibrillator (ICD) implantation markedly reduced the incidence of clinically significant pocket haematoma, as compared with the interruption of warfarin27. Of note, patients were bridged with either LMWH or intravenous heparin in the interruption group. In the COMPARE trial, patients undergoing catheter ablation for AF who continued warfarin had a lower risk of stroke and minor bleeding, as compared to interruption of warfarin and bridging with LMWH28. In the RE-CIRCUIT trial, AF ablation under continued dabigatran was associated with fewer bleeding complications than under continued warfarin29. Finally, continuation of OAC in patients undergoing coronary angiography with or without percutaneous coronary intervention also seems to be a safe approach30.
Accordingly, a recent series of retrospective analyses evaluated the continuation versus interruption of OAC during TAVI11121314. Continuation of OAC did not seem to increase bleeding or vascular complications. Also, transfusions were required less often in the continuation group. Moreover, an indication of lower stroke risk was observed in patients who continued OAC during TAVI1113. However, the observational design and retrospective character of these analyses, leading to a high risk of confounding and selection bias, hamper translation towards clinical practice15. For example, a trend towards increased inclusion over time was observed in the continuation group as opposed to the interruption group; this was likely associated with a lower procedural risk, due to ongoing technical refinements over the years. Also, as OAC prescription changed over time, patients in the continuation group were more likely to be on DOAC than VKA. Furthermore, 90% of the VKA-treated patients in the interruption group were bridged with heparin. Consequently, two-thirds of the interruption group did not truly interrupt OAC. In fact, based on the results of the BRIDGE trial, these patients were at increased risk of major bleeding17. On the other hand, DOAC patients in the continuation group omitted their DOAC the morning of the procedure and restarted 24 to 48 hours after TAVI. As a result, half of the patients in the continuation group did not truly continue OAC.
Considering that the thromboembolic risk is highest on the day of TAVI and the first postprocedural days, a true continuation strategy was adopted in the design of the current trial, for both VKA and DOAC patients. Furthermore, based on the results of the BRIDGE trial, we opted to compare a true continuation strategy with a true interruption strategy, without the addition of a third arm in which LMWH bridging is used17.
Limitations
A pragmatic, open-label design was chosen, which potentially introduces the risk of reporting and ascertainment bias. This risk is considered low, because a blinded clinical endpoint committee adjudicates all potential primary endpoints, which are prespecified according to the standardised VARC-3 criteria. Furthermore, the trial was powered to demonstrate non-inferiority based on the combined primary composite endpoint, rather than powering for a separate thromboembolic and bleeding co-primary endpoint. Hence, the obtained study data may be limited for drawing definitive conclusions regarding the risk of individual endpoints, like major bleeding or ischaemic stroke, per OAC strategy.
Conclusions
In TAVI patients with a concomitant indication for OAC, the optimal periprocedural OAC strategy is unknown. POPular PAUSE TAVI is a randomised clinical trial to study the effect of periprocedural continuation versus interruption of OAC on mortality, stroke, vascular complications and bleeding, as well as on quality of life after TAVI.
Conflict of interest statement
The authors have no conflicts of interest to declare with respect to this manuscript.

