Abstract
Aims: Patients with symptomatic heart failure following acute ST-elevation myocardial infarction (STEMI) received transendocardial application of bone marrow-derived mononuclear cells (BMC) to improve left ventricular (LV) function and clinical outcome.
Methods and results: Patients (n=12) with LV ejection fraction (EF) <45% and NYHA Class ≥II received NOGA-guided transendocardial injection of BMC into the infarction border zone 17.5±0.8 days following successful interventional revascularisation after STEMI. A matched control group (n=11) was generated from the source data of the previously published LIPSIAbciximab-STEMI trial. Primary and secondary endpoints were derived from comparisons of baseline vs. six-month follow-up cardiac magnetic resonance imaging (CMR) measurements and clinical assessments. Following cell therapy we observed a significant increase of EF (+7.9±1.5%, p=0.001) while the control group showed no change. This effect was driven by a reduction of LV end-systolic volume (ESV) by –27.5±6.5 ml (p=0.001); LV end-diastolic volume (EDV) and scar volu-me remained unchanged. A significant decrease of NYHA Class was found only in the cell therapy group (–0.75 vs. –0.18, p=0.04). Findings were also translated into enhancement of clinical assessments (rehospitalisation for decompensated heart failure, six-minute walk test, NT-proBNP levels).
Conclusion: The data suggest transendocardial injection of BMC can be used safely in patients with sympto-matic heart failure following acute STEMI. These prospective, preliminary data of a well-characterised, small cohort suggest efficiency compared to routine treatment.
Introduction
Patients with large STEMIs suffer from LV remodelling despite successful revascularisation. Cardiac cell therapy employing autologous BMC applied via the intracoronary route was previously shown to be safe but not sufficiently effective1,2. In order to improve efficiency different cell types or delivery routes are currently being tested. Small, single-centre studies suggest the transendocardial route of delivery for BMC to be efficient in chronic ischaemic cardiomyo-pathy3. We previously demonstrated the feasibility of transendocardial cell therapy in post-STEMI patients, yet the study design and a 30% reintervention rate due to in-stent restenosis prevented us from judging on efficiency4. Here we describe the results of the ALSTER (trAns-endocardiaL therapy at ST. GEoRg hospital)-Stem Cell trial, where we tested the effects of NOGA-guided, transendocardial cell therapy in patients following acute STEMI and subsequently reduced EF despite successful revascularisation. The results were compared to a matched control group of revascularised, post-STEMI patients with optimal standard therapy5. Serial CMR measurements, now accepted as the most accurate and reproducible imaging technique for quantitative assessments of LV function3, as well as clinical parameters served as endpoints.
Methods
STUDY POPULATION AND PROTOCOL
Figure 1A and Figure 1B depict study recruitment and follow-up. Between January 2009 and July 2010 all patients admitted to the cardiology department at St. Georg Hospital for acute STEMI were assessed regarding EF following successful revascularisation by primary percutaneous coronary intervention (PCI). Patients aged <80 years and baseline measurements of EF <45% (CMR) assessed at least one week after PCI with additional symptoms of heart failure (NYHA Class ≥II) as well as NT-proBNP levels >250 pg/ml despite successful revascularisation were eligible. The baseline CMR was realised 14.8±1.1 days after STEMI. Thrombus in the LV, a wall thickness of less than 5 mm in the area of planned treatment, higher grade aortic stenosis (> grade I), STEMI >21 days prior to percutaneous coronary intervention (PCI) and obesity with body mass index >35 kg/m2 were exclusion criteria.
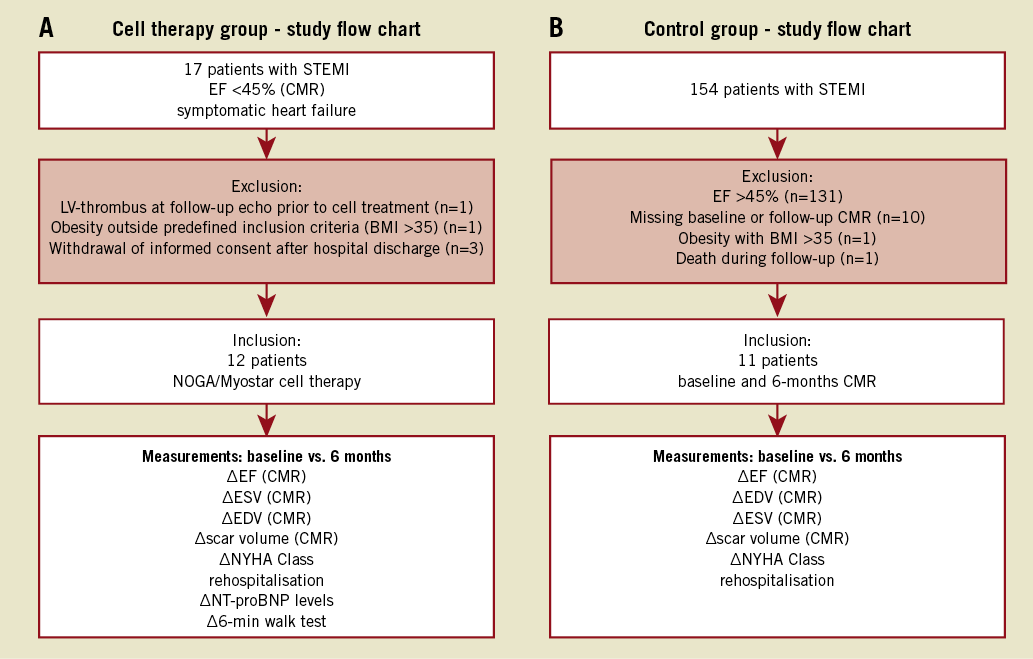
Figure 1. Study algorithm. A) Flow chart of cell therapy group. Five patients were excluded as displayed in the figure. For included patients follow-up was 100% complete at six months. B) Study flow chart of the control group, a subgroup of the LIPSIAbciximab-STEMI trial7. 143 patients were excluded due to various reasons presented in the figure.
Follow-up was performed three, six and 12 months after the stem cell procedure. NT-proBNP levels and patient history were taken at each visit to record possible hospital stays and other adverse events. Additionally, a six-minute walk test was performed at baseline and at six-month follow-up according to standard guidelines6. The CMR (measurements of EF, ESV, EDV and scar volume) and determination of NYHA Class were repeated at six months.
The control group was a subgroup of patients included in the LIPSIAbciximab-STEMI trial performed at the University of Leipzig Heart Center. The study evaluated the effects of intracoronary vs. systemic administration of abciximab during interventional revascularisation for acute STEMI. While the initial pilot study showed reduced infarct size, reduced extent of microvascular obstruction and improved perfusion in the intracoronary abciximab group, no effect on EF was detected5,7. Of note, the recently published large, multicentre AIDA STEMI trial testing this approach did not show any significant differences between the two delivery routes of abciximab8. Patients of the LIPSIAbciximab-STEMI trial received serial CMR measurements assessing LV function, dimensions and scar volume at baseline (2.6±0.5 days after STEMI) and at six months’ follow-up5,7. Using the source data of this trial a matched control group was generated. All patients with an initial EF <45% and both baseline and follow-up CMR at six months were included in the analyses performed in our study.
TRANSENDOCARDIAL CELL THERAPY
The NOGA platform (Biosense Webster, Inc., Diamond Bar, CA, USA) has been evaluated to identify regional myocardial ischaemia and tissue viability. Using point-by-point measurements it allows precise determination of the infarction border zone. The St. Georg approach regarding NOGA-guided transendocardial cell therapy following acute STEMI has been described previously4. In brief, patients were taken to the cardiac catheter laboratory 17.5±0.8 days after the index PCI for acute STEMI (Figure 2A). An amount of 150-200 ml of bone marrow was aspirated from the right upper iliac crest under local anaesthesia. Cells were collected into pre-heparinised syringes to prevent clotting during cell collection and subsequent cell processing. Bone marrow cells were mixed with 200 ml peripheral blood to increase the cell separation volume. The whole procedure of separa-ting the BM mononuclear cells from erythrocytes and platelets takes place under GMP conditions in the closed sterile system of the “Spectra therapeutic platform” (Gambro BCT, Lakewood, CO, USA) as described previously4. While BMC were prepared, mapping of the LV employing the NOGA platform was performed. In total 90-150 points were taken for mapping the whole LV. Unipolar voltage (UPV, infarction border zone=6-12 mV), bipolar voltage (BPV, infarction border zone=0.5-1.5 mV) and local linear shortening (LLS=regional wall motion, infarction border zone=6-12%) were all assessed to generate an electrophysiologically (UPV+BPV) and anatomically defined (LLS) map. These maps were used to draw a design line in the infarction border zone surrounding the ischaemic low UPV and/or BPV area (Figure 2C and Figure 2D). To identify mismatches between the border zones as defined by anatomical (LLS) and electrophysiological mapping (UPV+BPV) the maps of each patient were compared to each other. Mismatches were defined as areas demonstrated to be akinetic (LLS) but still partially electrically active (UPV+BPV, Figure 2C and Figure 2D). Using the NOGA® Myostar® injection catheter (Biosense Webster, Inc.) 15-20 injections of concentrated BMC (100 μl each) along the design line were performed; in total, 220±42*106 cells/patient were injected.
FACS ANALYSIS, PROLIFERATION AND MIGRATIONS ASSAYS
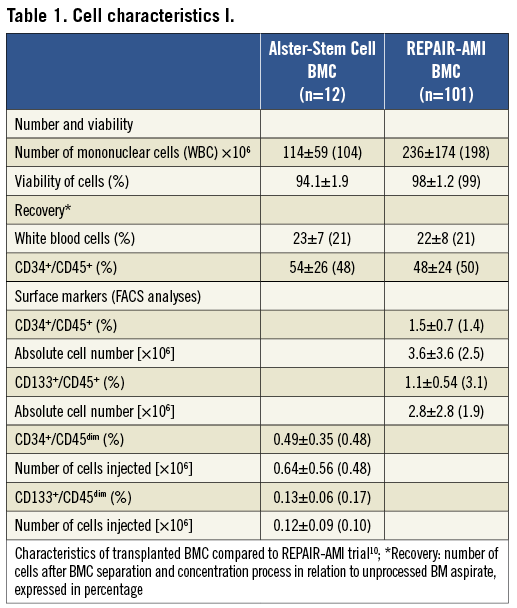
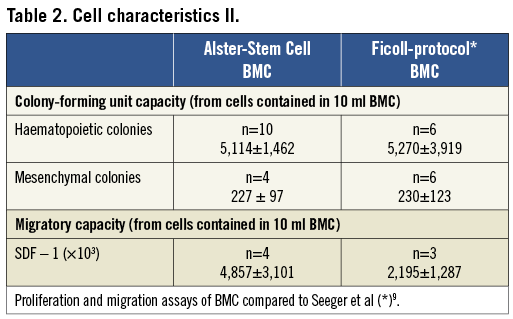
Aliquots of cells not injected were subjected to fluorescence activated cell sorting (FACS) analysis, proliferation and migration assays according to previously described methods9,10. To characterise the applied BMC we analysed different cell surface markers (CD34, CD45, CD133) by using FACS. Findings were compared to FACS data of the previously published REPAIR-AMI trial (Online Table 1)10. Findings of the proliferation and migration assays were compared to the data of Ficoll-isolated BMC published by Seeger et al (Online Table 2)9. Protocols and data of the FACS analysis, proliferation and migrations assays are provided in the supplementary appendix and show similar characteristics of the Gambro-isolated cells to the Ficoll method employed in REPAIR-AMI.
CMR measurements
Eligible patients underwent CMR diagnostics using contrast medium to examine LV function and infarction scar area. In detail, SSPF sequence analysis for the examination of LV function in the short axis model, analysis of the first pass perfusion and application of gadolinium-DTPA (0.1 ml/kg BW) for perfusion analysis as well as T1-weighted inversion-recovery gradient-echo sequence analysis were done for the demonstration of the “delayed enhancement” (fibrosis/necrosis).
STATISTICAL ANALYSIS
Statistical analysis was performed using GraphPad Prism version 4 (GraphPad Software, Inc., San Diego, CA, USA). Categorical data and rehospitalisation were analysed by the Fisher exact test. Predefined primary endpoint was the intra-individual change in EF (=ΔEF) as measured by CMR. Significance within each group (cell therapy, control group) was assessed by the Kolmogorov-Smirnov test followed by a one-sample t-test with “no change” (Δ=0) as the hypothetical value. Significance of ΔEF between the groups was analysed by the non-parametric Wilcoxon rank-sum test. Similar analysis was performed regarding the other secondary endpoints including NYHA Class, six-minute walk test, NT-proBNP levels and further CMR measurements (ESV, EDV, scar volume). A p-value <0.05 was considered to be statistically significant. All results are reported as mean±standard error of the mean.
Results
ENROLMENT AND PATIENT CHARACTERISTICS
For the cell therapy group, 17 patients were identified who fulfilled the entry criteria and gave written informed consent to enter the screening phase. Five patients were excluded for various reasons (Figure 1A). The remaining 12 patients were treated as described above. One patient was rehospitalised due to decompensated heart failure; he was successfully treated by medication. Furthermore, one patient was readmitted eight months after cell therapy for elective coronary angiography with PCI of the target vessel due to an 80% de novo stenosis: the vessel showed TIMI III flow at baseline. No other adverse cardiac events including repeat revascularisation or rhythm abnormalities were found.
A subgroup of patients included in the LIPSIAbciximab-STEMI trial was used as a control group. The trial itself comprised 154 patients. The ALSTER-Stem Cell inclusion criteria (EF <45% after successful PCI for acute STEMI, follow-up CMR at six months available) excluded 93% of patients (Figure 1B)7. The remaining 11 patients were used as a matched control group for the present study. Two patients were rehospitalised for decompensated heart failure, while one patient was readmitted for re-PCI of the target vessel. However, no reinfarction occurred either in the cell therapy or in the control group within the one-year follow-up period. No prior infarction was documented concerning the cell therapy group, while in the control group one patient had such an incident before. That prior infarction did not lead to reduced EF and was located within a different vessel.
BASELINE CHARACTERISTICS
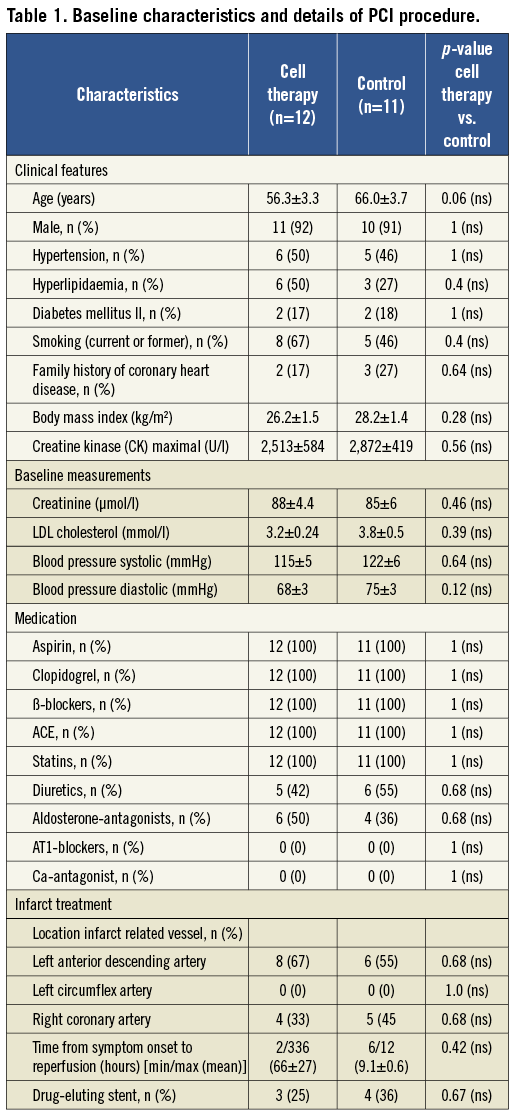
Patient baseline characteristics are shown in Table 1. The two groups were well matched and similar concerning baseline characteristics. The cell therapy group showed a trend towards younger age (56.3±3.3 vs. 66.0±3.7 years, p=0.06). A majority of patients (66.7%) in the cell therapy group presented late to the hospital with a delay of ≥6 h from symptom onset. Seven patients (58.3%) had a delay greater than 12 h certainly contributing to the reduced EF and large infarctions (creatine kinase [CK] max=2513±584 U/l) despite successful PCI. All control group patients presented between 6-12 h after symptom onset. Door to needle time was equal in both groups (<1 h). Microvascular obstruction, known as an indicator for high risk with poor outcome and rapid LV remodelling, was detected in 6/12 of cell therapy patients by CMR11,12. Data for the control group regarding microvascular obstruction were not available. CKmax levels of the control group were equally high (2872±419 U/I, p=0.56).
INTRAPROCEDURAL MEASUREMENTS
There was an UPV-LLS mismatch in 9/12 patients while 10/12 patients presented a BPV-LLS mismatch. The LLS-defined akinetic or hypokinetic area was always larger than the unipolar and/or bipolar defined scar area. Figure 2C and Figure 2D depict an exemplary image for a BPV/LLS mismatch. The border zone as detected by electroanatomical mapping was similar to areas with delayed enhancement as detected by CMR (Figure 2B).
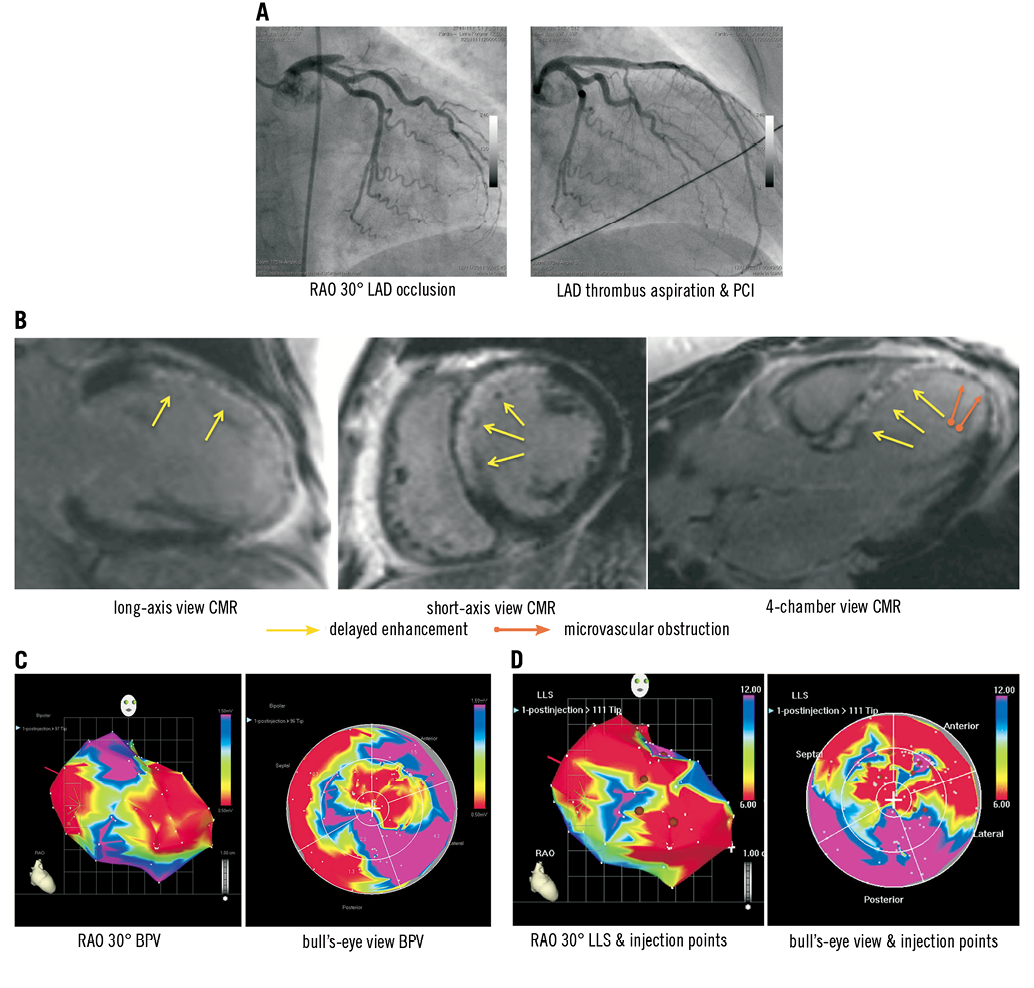
Figure 2. Exemplary images of a 51-year-old woman with proximal left anterior descending artery occlusion and successful revascularisation by PCI three to four weeks after STEMI. A) Angiographic details at baseline and after PCI including thrombus aspiration and eptifibatide. B) CMR images 10 days after STEMI. Note the marked delayed enhancement showing oedema and early fibrosis as well as microvascular obstruction in the apex. C) Bipolar voltage (BVP) and D) local linear shortening (LLS) mappings in RAO 30° and bull’s-eye projections are displayed with margins manually set to standardised values (BPV=0.5-1.5 mV, LLS=6-12%). A mismatch between BPV as well as regional wall motion (LLS) is observed, with the hypokinetic area (LLS) larger than the “electrophysiological” (BVP) scar area. White points indicate mapping points; brown dots indicate locations for injections in the infarction border zone as defined by a bipolar signal of 0.5-1.5 mV (alternatively UPV of 6-12 mV). Colour encoding is displayed at the right upper border of the pictures. Valve area at the heart base is electrical inactive but with positive movement (LLS signal). The exemplary images are all displayed in the same projection (RAO 30°).
High thrombus burden led to fibrosis, necrosis and microvascular obstruction in the CMR analysis 14.8±1.1 days after revascularisation. The mapping demonstrates the transmural scar (red area, BPV <0.5 mV), the coloured border zone (BPV=0.5-1.5 mV) as well as normal myocardium (purple area, BPV >1.5 mV, Figure 2C).
QUANTITATIVE MEASUREMENT OF LV FUNCTION
Patients treated with cell therapy showed an intra-individual improvement of EF by Δ=+7.9±1.5% (p=0.001). In contrast, EF did not change in patients of the control group (Δ=+0.6±1.9%, p=0.29). This difference reached statistical significance in the intergroup comparison (p=0.008). In four patients with an EF <35% at six months’ follow-up an internal cardioverter defibrillator was implanted for primary sudden cardiac death prophylaxis.
Cell therapy patients showed a significant decrease of ESV (ΔESV=–27.5±6.5 ml, p=0.001), while control group patients had no change (-0.85±10 ml, p=0.4; p=0.02 for the intergroup comparison). Patients with microvascular obstruction as detected by CMR showed less but still significant EF improvement (ΔEF=+5.8±1.7 vs. +10±2.4%). The cell therapy group showed less LV dilation after six months (ΔEDV=–4.8±12.1 ml, p=0.31), whereas the control group marginally increased EDV by Δ=+1.1±11.1 ml, p=0.42.
In order to explain the improved EF mechanistically we analysed the change in scar volume. The cell therapy group showed no significant change between baseline and follow-up measurements (Δ=–0.05±3.5 ml, p=0.28), while control group patients showed a decrease of scar volume (Δ=–4.0±2.0 ml, p=0.01; p=0.52 for the intergroup comparison). Findings are presented in Table 2 and Figure 3.
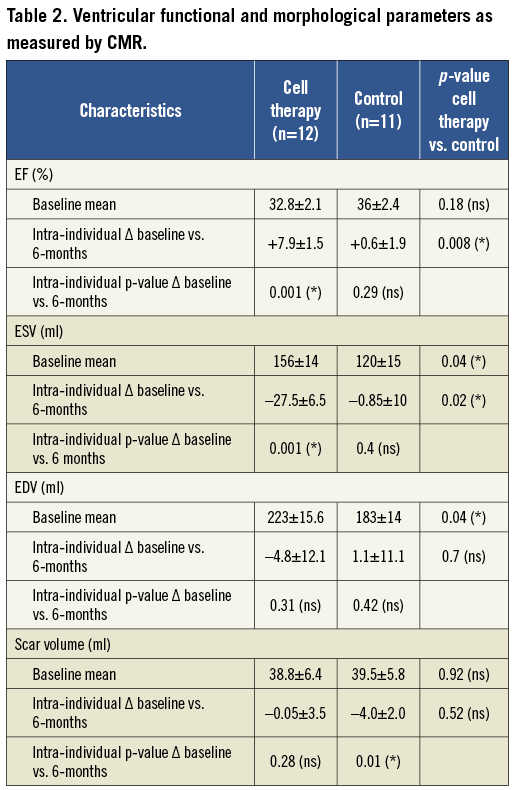
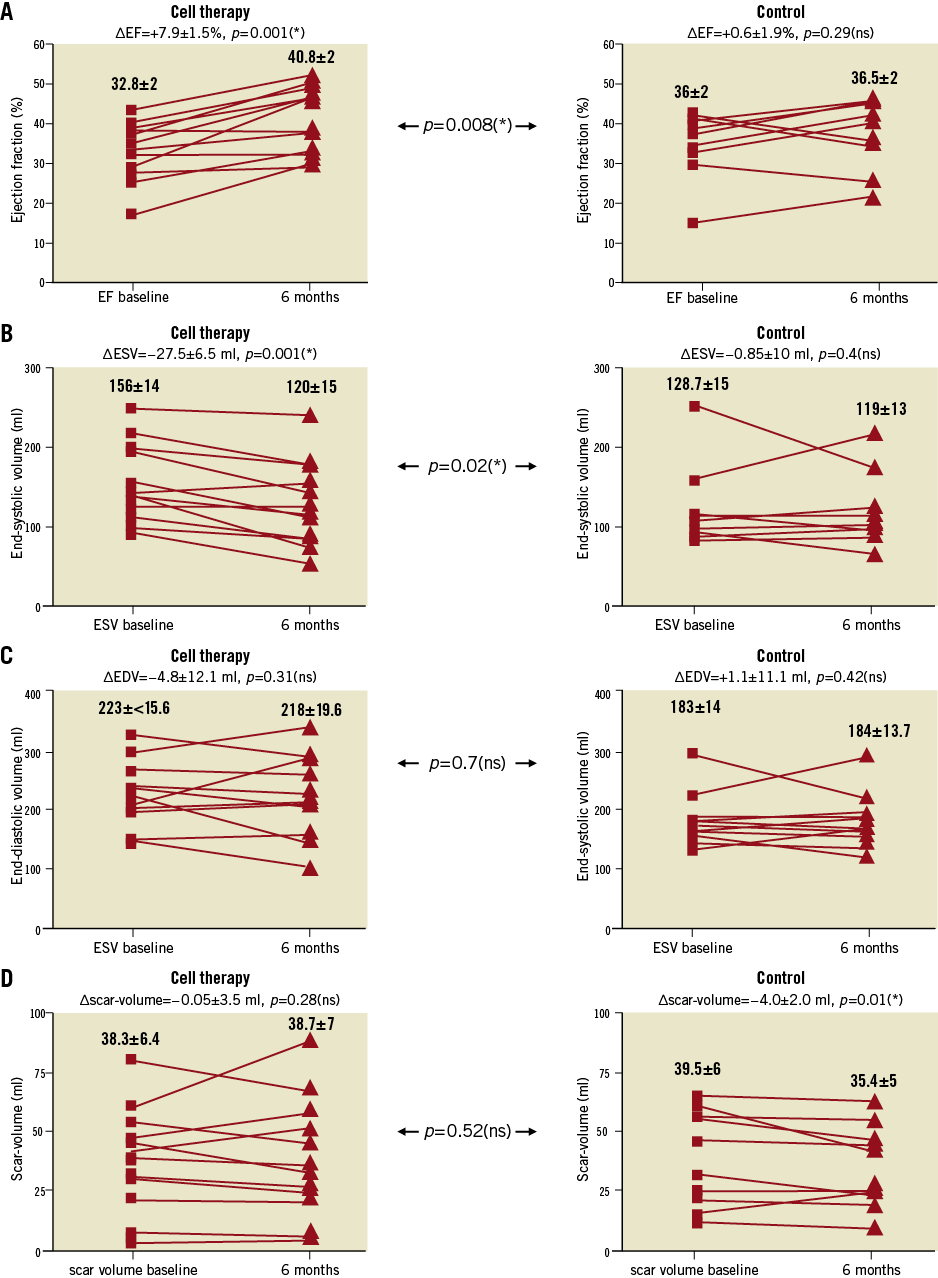
Figure 3. CMR data regarding EF (A), ESV (B), EDV (C) and scar volume (D), at baseline vs. six months’ follow-up. Small numbers indicate mean±standard error of the mean. An increase in EF following PCI plus injection of autologous BMC over six months was observed (A, cell therapy). Compared to control (A, control), the change was significant in an intergroup analysis regarding ΔEF. Findings are accompanied by a significant decrease of ESV in the cell therapy group (B); no significant effect on ESV was observed in the control group (B, control). No significant change in EDV and scar volume was observed over the six months time course (C+D, cell therapy).
CLINICAL OUTCOME
Patients undergoing cell therapy showed an average NYHA Class of 2.2±0.1 at baseline, while control group patients started at 1.7±0.2 (p=0.14). The intra-individual analysis (baseline compared to follow-up) revealed a significant reduction in NYHA Class in the cell therapy group (Δ=–0.75±0.13, p=0.0001), which was not observed in the control group (Δ=–0.18±0.2, p=0.28). The intergroup analysis found this difference to be significant (p=0.04, Table 3).
Concerning one-year rehospitalisation for decompensated heart failure one patient of the cell therapy group and two control group patients were readmitted. The comparison between the groups revealed no significant change, yet there was an absolute difference favouring the cell therapy study group (p=0.59, Table 3).
NT-proBNP levels were analysed concerning the cell therapy group while these data were not available for control group patients. Measurements were conducted at baseline and three, six and 12 months after stem cell injection, showing significant improvements among all investigated time points (Figure 4A). As a further endpoint a six-minute walk test was realised in the cell therapy group (Figure 4B) showing a significant improvement (baseline vs. six-month follow-up) of +100.3±20.7 metres, p=0.04.
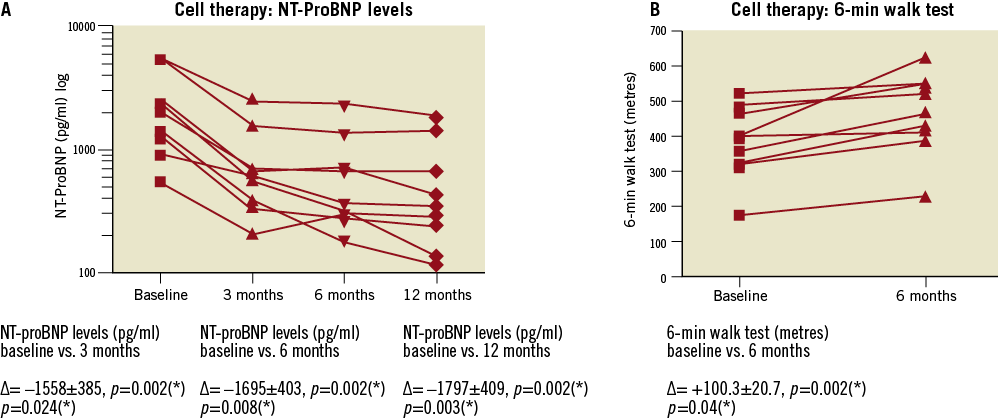
Figure 4. Parameters of heart failure in the cell therapy group. A) Sustained and significant decrease of NT-proBNP levels in the cell therapy group from baseline to follow-up at three, six and 12 months. Data were not available for the control group. B) Significant intra-individual increase in symptom-free walk distance as measured by six-minute walk test in the cell therapy group patients from baseline to follow-up at six months. Data were not available for the control group.
Discussion
We find NOGA-guided, transendocardial injection of BMC 17.5±0.8 days following acute STEMI is feasible in an experienced centre, well tolerated and possibly effective. Clinical assessments improved over a one-year follow-up time period despite the included patient cohort being at high risk for LV remodelling (baseline EF <45%, large infarctions with delayed reperfusion, proven microvascular obstruction)11-13. Except for the reported cases (one elective PCI and one rehospitalisation due to decompensated heart failure) no adverse cardiac events were found concerning the cell therapy group. Additionally, NT-proBNP levels and six-minute walk test improved significantly. As these parameters are not avai-lable from the control group they may suggest efficiency of the stem cell treatment but may also be dually attributed to a training effect or spontaneous recovery.
The study used CMR measurements as the primary endpoint, currently denoted as the most accurate and reproducible imaging technique3. Statistical analysis of data was based on an intra-individual comparison baseline vs. follow-up, where each patient served as his internal control. On a single-centre basis we were not able to recruit enough patients with an EF <45% three weeks after the infarction despite succesful PCI. This observation led to the formation of a matched control group with the below-mentioned impairments.
In order to verify the identity and viability of transplanted cells FACS analysis as well as proliferation and migration assays were performed. We suggest that this procedure is pivotal for interpreting the results of cell therapy studies in general. In our trial findings of FACS analysis concerning viability, recovery as well as cell surface marker analysis were related to the data of the previously published REPAIR-AMI trial10. Furthermore, data of proliferation and migration assays were comparable to the findings published by Seeger et al9. We therefore assume that cells used in the present study show a sufficiently high paracrine capacity in order to explain our observations.
A mismatch between the electrophysiologically and anatomically defined border zones was observed. Most likely this mismatch represents infarction areas with non-transmural scars yet sufficient damage to affect contractile function. The delayed CMR in the cell therapy group should exclude stunning as the underlying phenomenon; the lack of scar-volume reduction as detected by CMR between baseline and six months’ follow-up also supports this interpretation.
By exclusion of other possible mechanisms we believe that true myocardial regeneration contributes to improved EF in our cohort. No significant effects on EDV or scar volume were detected, while improvement of EF was accompanied by a decrease of ESV. Stunning of the myocardium can be excluded as the initial CMR was performed 14.8±1.1 days after successful revascularisation. The increased EF after cell therapy could also have resulted from attenuated LV remodelling; yet neither the control nor the cell therapy group demonstrated significant changes of EDV over the six months. Furthermore, reduction of scar volume does not explain the difference in EF, because no change of scar volume was observed after cell therapy. Control group patients showed a slight reduction of scar volume, while further CMR parameters did not change. This phenomenon could be explained by the relatively early implementation of CMR in the control group compared to the cell therapy group (2.6±0.5 vs. 14.8±1.1 days after STEMI).
The recently published CADUCEUS trial suggests that myocardial regeneration is possible in post-myocardial infarction patients. Using autologous stem cells derived from endomyocardial biopsy and subsequent intra-coronary application, a significantly reduced scar volume was observed. Nevertheless, CMR measurements including EDV, ESV, EF did not differ compared to control14. Compared to our own findings the different delivery route could be responsible for this discrepancy. We propose enhanced endogenous regeneration from resident cardiac progenitor cells (CPC) by targeted BMC administration to the infarction border zone as the most likely mechanism behind EF improvement in our cohort15.
Interestingly, patients with microvascular obstruction showed less improvement of EF compared to patients without it. However, even patients with proven microvascular obstruction demonstrated improvement of EF and clinical outcome. Microvascular obstruction and low EF could possibly serve as a screening tool to select patients eligible for transendocardial cell therapy which, due to its sophisticated technique, will only be practical for a minority of STEMI patients at high risk for heart failure due to LV remodelling. On the other hand, large infarctions with the described phenomenon of NOGA-detected mismatch between electrophysiological and anatomical defined scar may be specifically amenable to this kind of treatment. In this trial no correlation between the electro-anatomical mismatch identified by NOGA map and findings of further investigations could be found. The sample size is too small to perform subgroup analyses. Nevertheless, in further upcoming studies it would be interesting to examine whether mapping results are able to predict the outcome of cell therapy.
The randomised, multicentre, placebo-controlled LateTIME trial employing a similar closed loop cell preparation system and CMR endpoints was not able to find a positive effect of intracoronary cell therapy two to three weeks after MI regarding primary or any secondary endpoints2. Interestingly, inclusion criteria (EF <45%), age (ALSTER-Stem Cell: 56.3±3.3 years; LateTIME 57.6±11 years) and time course of treatment (>2 weeks after PCI for STEMI) were very similar between the ALSTER-Stem Cell trial and LateTIME, yet outcome was completely different. While intracoronary BMC treatment had no effect on EF, transendocardial BMC therapy improved EF in our study2. The small sample sizes both in the cell therapy as well as in the control group prevented us from drawing definite conclusions. However, baseline EF and time from chest pain to PCI was different in LateTIME compared to our study (48.7% and 3 h, respectively). We suggest an EF <45% outside the stunning phase of approximately seven days and/or microvascular obstruction as analysed by CMR might be an important screening tool to identify patients who apparently profit from transendocardial BMC application.
Several small single-centre trials have found positive results with transendocardial delivery in patients with chronic ischaemic cardio-myopathy employing the NOGA® Myostar® and other systems16,17. Pokushalov published the data of the, to date, largest series of ischaemic heart failure patients treated with NOGA-guided BMC: this study was a randomised, mono-centre trial including patients with EF <35% and NYHA Class III-IV. Clinical data including mortality suggest the treatment to be effective18. Whereas this approach showed positive results, the recently published FOCUS-HF trial could not find any effect of transendocardial injection of BMC in patients with chronic ischaemic cardiomyopathy (NYHA II-IV, EF <45%)19.
As a consequence of this conflicting data various groups in the cardiac cell therapy field have recently focused on alternative, possibly more efficient cell types compared to BMC. These include, but are not limited to, bone marrow-derived cardiopoetic cells20, adipose-derived stem cells21, cardiosphere-derived cells22, allogenic mesenchymal precursor cells23, CD34+ cells24 and CD133+ cells25. The still on-going phase I open label randomised SCIPIO trial used intra-coronary infusion of ckit+ cardiac stem cells (CSC) in patients with ischaemic heart failure with EF <40% before coronary artery bypass grafting. No CSC-related adverse events have been reported to date and EF increased from 30.3% to 38.5% at four months after cell therapy, while the control presented no change of EF26.
It is now uniformly accepted that BMC and derivatives like CD34+ cells are partially effective in amplifying endogenous angiogenesis yet true myocardial regeneration is still an issue of debate3. Autologous BMC can be prepared by closed-loop systems at the bedside; this has several advantages also from a regulatory point of view. Head-to-head trials of the new cell sources with crude, autologous BMC are warranted to demonstrate the possible superiority of these more specific cells.
Paracrine mechanisms enhancing cardiac-endogenous regeneration rather than transdifferentiation of transplanted cells are now accepted to be the key mechanisms for myocardial regeneration. However, the precise factors involved in paracrine induction of regeneration as well as cellular markers of cardiac-endogenous progenitor cells are unclear to date. Recently, BMC were found to be more efficient regarding secretion of paracrine factors enhancing endogenous cardiac regeneration compared to mesenchymal stem cells27. Therefore, paracrine mechanisms initiated by the injected BMC could be responsible for the positive effects observed in the present and further trials.
Our study is limited by its small sample size, the lack of a randomised control group as well as a predefined power calculation and variations in patient baseline data. Nonetheless, the patient characteristics were statistically similar in both groups. The present data is not able to exclude the differences in baseline EDV and ESV as well as diverse time points of CMR measurements, which may have influenced the outcome over the follow-up period. Observations of clinical outcome, regarding NT-proBNP levels and six-minute walk test were only available for the cell therapy group. These parameters were measured just prior to the cell therapy procedure, approximately three weeks after PCI for STEMI. We cannot exclude training effects or spontaneous recovery being responsible for our findings. Yet again, patients with microvascular obstruction have shown clinical deterioration with increased NT-proBNP28, still we regard the trend we observed in this special patient cohort as being remarkable.
In summary, our data support the hypothesis that NOGA-based transendocardial delivery of BMC in patients with symptomatic heart failure after acute STEMI is safe, feasible and possibly effective regarding improvement of EF. In relation to the discrepant findings of the latest publications, the effectiveness of this sophisticated approach needs to be proven in larger, randomised phase III studies or registries.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Table 1. Cell characteristics I.
Table 2. Cell characteristics II.
MESENCHYMAL STEM CELL COLONY-FORMING UNIT ASSAY (MESENCULT ASSAY)
Separated BMC (2×107) were suspended in 10 ml MesenCult (Art. -Nr. 05411), plated on a T25 culture flask, and cultured at 37°C. After 3.5 days the medium was changed for the first time and then every second day. After 12 days colonies were counted using phase contrast microscopy.
HAEMATOPOIETIC STEM CELL COLONY-FORMING UNIT ASSAY (METHOCULT ASSAY)
Separated BMC (1×105 per dish) were seeded in methylcellulose plates (MethoCult GF H4534). Plates were examined using phase contrast microscopy, and colony-forming units (CFU; colonies >50 cells) were
counted after a 14-day incubation period at 37°C by independent investigators. CFUs were assessed in triplicates.
MATRIGEL INVASION ASSAY (BOYDEN CHAMBER)
Separated BMC (1×106) were re-suspended in 250 µl X-VIVO™ 10 medium and placed in the upper chamber of a modified Boyden chamber filled with Matrigel (BioCoat invasion assay, 8 mm pore size). The chamber was placed in a 24-well culture dish containing 500 µl endothelial basal medium supplemented with 20% foetal calf serum and 100 ng/ml SDF-1. After 22 h of incubation at 37°C, transmigrated cells were counted by independent investigators. Invasion assays were run in duplicates.