Abstract
Aims: To assess the hypothesis that fluoroscopically-guided helical needle transendocardial delivery of autologous bone marrow (ABM) mononuclear cells (MNCs) in chronic post myocardial infarction patients is safe and improves ejection fraction (EF).
Methods and results: Twenty ischaemic heart failure patients with an EF ≤40% were enrolled. ABMMNCs were prepared, counted for CD34+ and CD133+ content, and delivered percutaneously to the heart at 5 to 10 peri-infarct sites. Two-dimensional (2D) transthoracic echocardiography, EF measurements, Holter, and exercise tolerance time (ETT) were performed at baseline, one week (wk), and 6, 12, and 24 months (mo). 96±29 million ABMMNCs were injected into 8.5±2.6 peri-infarct sites over 42±17 minutes (n=20). There were no adverse events associated with the catheter-based cell transplantation procedure or significant increases in ventricular events on Holter. EF improved over baseline from 34.9±4.3% to 41.9±5.1% at 12mo to 42.2±7.1% (p =0.00005) at 24mo. ETT improvements were statistically significant from 246±113 sec to 373±183 sec at 12mo and 371±181 sec at 24 mo (p=0.006).
Conclusions: ABMMNCs delivered with the helical needle transendocardial catheter was safe in this uncontrolled open label study. Increased EF and ETT support the safety of the procedure and technologies involved and warrant additional investigation.
Introduction
Cardiac cell therapy clinical studies using autologous bone marrow mononuclear cells (ABMMNC) have been performed in a surgical setting for ischaemia and chronic infarction1,2, with intracoronary artery (IC) catheters for acute myocardial infarction (MI)3-7, or percutaneously with transendocardial delivery catheters for ischaemia and heart failure8-10. At this time, cumulative data from more than two thousand patients treated suggests that this therapeutic strategy is safe regardless of administration method used. There are inconsistent efficacy results from the IC studies performed to date, due possibly to either the lack of statistical power, differences in timing of delivery after MI onset, suboptimal cell processing, or the poor retention associated with the IC route of delivery4-7. Conversely, all clinical studies with intramyocardial delivery of ABMMNCs have had consistently positive safety and efficacy results.
The authors herein present two-year safety data with the only helical transendocardial intramyocardial delivery (TIMD) system in use in clinical trials. The purpose of the present study was to assess the safety of a helical TIMD system for the delivery of ABM to multiple segments of the peri-infarct region in a setting of chronic MI. The patients were stable and had EF of ≤40%. This differs from earlier studies in: (1) the TIMD system used, (2) the patients selected having chronic ischaemic heart failure and were also relatively stable as compared to the existing data sets in patients with acute MI, and (3) the targeting of the cell delivery to the distal border regions. A first cohort of ten patients was previously reported11. A second cohort of 10 patients was similarly treated, and all twenty subjects have been followed for two years.
Methods
This study was approved by the Argentina National Institute for Coordination of Ablation and Implantation; the Argentina National Administration for Medication and Medical Technology; and the institutional review committees at both the Instituto Argentino de Diagnostico y Tratamiento and Clinica Suizo Argentina. All patients gave their informed consent.
Population studied
Patients who were enrolled had sustained a presumed transmural myocardial infarction and showed echocardiographic evidence of a left ventricular dysfunction, having ejection fractions of ≤40%. Baseline evaluations included informed consent, history and physical examination, electrocardiogram, 24-hour Holter monitoring, echocardiography, routine blood tests and exercise tolerance testing. Transmurality of infarction was assessed based on a past history of a classic myocardial infarction, with new Q-waves developed in serial ECG, and orthogonal angiographic view of the left ventricle. Akinesis of the walls were coincident with the diseased coronary vessel in each patient.
Marrow harvest and processing
Approximately four hours prior to the percutaneous catheterisation, 50 ml of autologous bone marrow cells were collected under local anaesthesia from the posterior iliac crest. Bone marrow mononuclear cells were isolated by density gradient on Ficoll-Paque Plus tubes (GE Heathcare, Chalfont St Giles, UK). Cells were washed and filtered through 100 µm nylon mesh to remove cell aggregates, and re-suspended in Ringer’s solution at a concentration of 1x108 cells/ml in a total volume of 1.3 ml. Ten percent of the final cell solution was used for cell counting, flow cytometry analysis, and viability testing using trypan blue exclusion.
Catheter delivery of cells
Prior to cell delivery, the infarct target zones were defined using ECG, baseline echocardiography, and previous orthogonal ventriculography data. Regions of infarct were agreed upon by the clinical team prior to catheterisation. Decisions of target zones were specified based on a “bulls eye” map in which the left ventricle is divided into apical, medial, and basal transverse sections. Each section in turn was divided into eight sectors. Distal sections were targeted preferentially as explained previously11. Upon catheterisation, the selected target regions were assessed using fluoroscopy. After each fixation of the catheter to the heart wall, and prior to each delivery, three investigators established anatomic consensus regarding the helical needle placement in predefined target areas around the agreed upon regions of infarction. This was recorded by a technician on a “bulls eye” map.
Cells were delivered transendocardially with the Helical Infusion System (BioCardia Inc., San Carlos, CA, USA) (Figure 1). This TIMD device platform is comprised of a two catheter system: the transendocardial helical infusion catheter (Helix) tipped with a hollow helical needle, and the Morph® universal deflectable guide catheter (Morph). The Morph provides for distal tip deflection with a variable radius of curvature while retaining a large lumen that enables advancement of the Helix. The Helix extends out from the Morph guide and then engages the endocardium by having the helical needle rotated into the muscle. This helical needle engagement into the myocardium is similar to active fixation pacing leads that have been used clinically in more than a million patients. This provides stable engagement with the heart wall during therapeutic delivery11. The helical needle is mounted on a flexible coil shaft (also similar to implantable cardiac lead designs) and reduces the amount of force exerted along the catheter axis with each heartbeat mitigating the likelihood of cardiac perforation. The Morph is advanced through the aortic arch and aortic valve over a guidewire, and allows for rotation, and tip shape change within the heart. The Helix TIMD catheter, which passes through this Morph catheter, is advanced axially to reach out to the heart wall. It is then rotated clockwise two to three turns into the heart wall to provide for stable engagement during delivery to a depth of up to 4 mm within the myocardium. Unlike other unibody steerable TIMD catheter systems with two degrees of freedom, the Helical Infusion System has three independent degrees of freedom (deflection, rotation, reach), or four degrees if one includes rotation of the Helix into the myocardium.

Figure 1. The Helical Infusion System has three dimensional mechanical control (1=rotation of the Morph guide shaft, 2=deflection of the Morph guide distal element, and 3=reach of the helical needle to the heart wall). This is a dual lumen catheter that enables stable TIMD into the heart through the hollow helical needle while engaged, and confirmation of engagement through the delivery of small volumes or contrast at a port on the distal end of the coil shaft and at the base of the helical needle.
This delivery protocol included: (1) confirmation of location within the ventricle by fluoroscopy, with contrast position confirmation through a second lumen at the base of the helix, (2) a fifteen to thirty second infusion time while engaged in the heart wall, and (3) a fifteen to thirty second dwell time aimed at enabling the penetrating Helix to act as a barrier to back-leak of the cells into the ventricular chamber. After the dwell period, the helical needle catheter was rotated counter clockwise five or more turns and repositioned for another delivery or removed.
In the first five patients treated, it was intended to deliver up to five 0.1 to 0.2 ml injections of cells at a concentration of 1×108 cells/ml. In the subsequent fifteen patients, the intention was to deliver up to ten 0.1 to 0.2 ml injections at up to ten targeted sites in each patient using a maximum dose of 1.2×108 cells.
No ACT measurement was done during the procedure. Standard clinical practice to keep the ACT at 250 seconds for routine coronary procedures was followed, which includes dosage of heparin of 100 U/Kg. Further, a heparised saline of 2000U/litre was connected to the Morph guide at a rate of 15 to 20 drops per minute.
Post-procedure clinical assessments
Baseline and post-transplant clinical assessments included: monitoring of adverse events and all concomitant medications, physical examination, ECG, echocardiography, 24-hour Holter monitoring, exercise tolerance testing, blood testing, and urinalysis. Here, we report two-year follow-up on twenty patients enrolled in two cohorts at baseline, 1 week, 6 months, 12 months, and 24 months.
Statistics
All p-values reported are from paired two-tailed t-tests. Average values are presented with standard deviations.
Result
Patient population
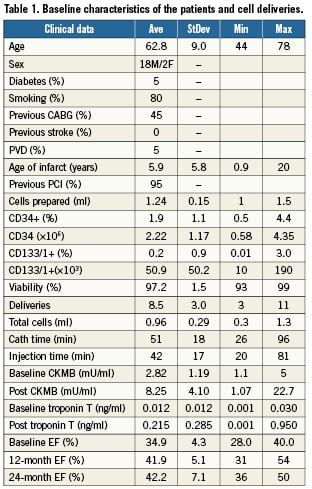
Patient demographics for the twenty patients enrolled are shown in Table 1. All infarcts treated were greater than 11 months old with an average of almost six years. Baseline ejection fractions ranged from 28% to 40% with an average of 34.9±4.4%.
Procedures
From the 50 ml of marrow aspirated from each of the twenty patients on the morning of their respective procedures, 1.24±0.15×108 cells were prepared for delivery at a concentration of 100 million cells per ml. Of these cells, 97.2%±1.5% were deemed viable as determined by trypan blue exclusion assay.
The first five patients were injected at three to five targeted delivery sites. As no major adverse event was noted, fifteen additional patients received nine to eleven TIMDs. Deliveries were recorded on a “bulls eye” myocardial location code chart at the time of delivery. No incidences of myocardial perforation, stroke, or myocardial infarction resulted from a total of 170 injections. At each penetration of the myocardium there were premature ventricular contractions, but no sustained arrhythmias or runs of ventricular tachycardia occurred.
Early safety and serious adverse events
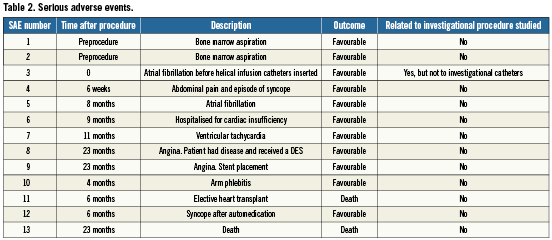
No complications or adverse events from the delivery device or cell transplantation procedure were observed. All patients were monitored at the hospital for 24 hours prior to being discharged. All serious adverse events (SAE) are reported in Table 2 organised in order of their occurrence with correlation to their time after procedure. Only one SAE occurred during the procedure related to the insertion of a non-investigational angiography catheter.
Early safety was assessed by ECG changes at discharge, absence of pericardial effusion as assessed by echocardiography post procedure, and the detection of ventricular tachycardia runs at one-week follow-up, as discussed below. For every patient, ECG at discharge showed either no change as compared to baseline, or a change evaluated by an investigator as “non-clinically significant”. An echocardiography performed post procedure showed no patient had cardiac tamponadeand no patient had clinical, angiographic, or echographic signs of perforation.
Adverse cardiac events observed consisted of an episode of ventricular tachyarrhythmia at 11 months after the procedure for which an AICD was electively implanted (patient 3), an episode of atrial fibrillation four months after the procedure (patient 8), and an episode of heart failure requiring hospitalisation three months after the procedure (patient 9). The patient who received the AICD did not demonstrate any significant Holter detected arrhythmias at baseline or follow-up. This patient has also experienced no AICD discharges since implantation; a period of over five years.
Two patients died during the course of follow-up to this study. Patient 11 died during an elective heart transplant 177 days after the procedure. He had presented with a severe ischaemic cardiomyopathy at the time of treatment and had previously been enrolled for heart transplant. After the one-week follow-up visit, the patient returned to his home country. A donor heart became available six months after the stem cell procedure, so the subject elected transplant, but expired during the surgery. Pathology revealed diffuse scarring with fibrosclerosis throughout the thickness of the myocardium including the subendocardium. Marked loss of cardiomyocytes was noted. At the apex of the left ventricle, surgical foreign body material was observed, with marked surrounding fibrosis. The sections of the coronary arteries showed features of atherosclerosis with thickening of the intima, cells loaded with cholesterol and some foci of calcification. The final diagnosis was “diffuse and multifocal fibrosclerosis, possibly related to chronic ischaemia”. This patient had undergone previous ventricular resection receiving a patch, which explained the presence of foreign body material. Patient 16 died 695 days after stem cell injection from unknown causes. It had been nearly a year since he had last been seen by the investigators and no post-mortem analysis was performed.
Several non-cardiac adverse events also occurred, bringing to 13 the total number of adverse events in this study. All but two (the patient deaths) had a favourable outcome, and none are considered related to the procedure.
Cardiac enzymes and laboratory parameters
In order to measure the result of transendocardial tissue penetrations, cardiac enzymes (both non-specific CPK and LDH, and specific CPK-MB and troponin T) were measured one hour after delivery. CPK MB levels rose significantly from an average of 2.8±1.2 mU/ml to 9.3±4.7 mU/ml (normal range below 6) and troponin T levels rose significantly from an average of 0.014±0.013 to 0.23±0.28 ng/ml (normal range below 0.03, considered intermediate between 0.03 and 0.1, and positive above 0.1). Both non-specific enzymes CPK and LDH did not show any significant elevation and remain in the normal range (under 290 for the CPK and under 450 for the LDH) reported by our centres. Other laboratory parameters such as haematologic, hepatic and renal function did not show any alteration as a result of the intervention.
Electrocardiogram and Holter analysis
An ECG was performed at each visit and its qualitative analysis was recorded in the case report form by the investigator. Besides the adverse events described above, no significant change in the electrocardiograms was recorded. At 24 months, no sustained arrhythmias were noted in any patient.
All patients were monitored by a 24-hour Holter recording at baseline, 1 week, 6, 12 and 24 months. The number of ventricular arrhythmic events in a 24-hour period is presented in Figure 2. The frequency of ventricular arrhythmic events at follow-up is not statistically different from baseline. At two-year follow-up, patient 18 had significant premature ventricular contractions, which included a VT run of five contractions. This one patient has contributed significantly to the increased average in ventricular extra systoles at two years.
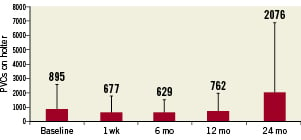
Figure 2. Average ventricular premature contractions on 24-hour Holter. There was no significant difference between baseline and any follow-up visit. Error bars show standard deviations.
Echocardiography: left ventricular function
Echocardiography was performed at baseline and at every time point as both a measure of safety and ventricular function, as well as to provide insights into potential benefits of the therapy. No persistent pericardial effusion or worsening of ventricular function was observed. The average value of all patients at all time points show an increase in left ventricular ejection fraction over baseline, suggesting potential benefit of the cell therapy (Figure 3). The difference of average ejection fraction is statistically significant over baseline at all time points (two-tailed paired t-test) with p-values as compared to baseline of less than 0.05 (0.03, 0.00002, 0.000006 and 0.00005 for the time points 1 week, 6 months, 12 months and 24 months, respectively). Figure 4 shows the left ventricular end-diastolic volume measured by transthoracic echo showing a decreasing trend that is not significant even at 24 months (p=0.07). No data on intra- and inter-observer variability were obtained. Operators were not blinded to the patients’ involvement in the study or to the time course of the echocardiographic measurements made.
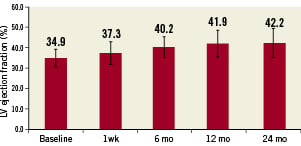
Figure 3. Average left ventricular ejection fraction as measured with transthoracic echocardiography showing a statistically significant improvement over baseline at all follow-up periods. p=0.03, 0.00002, 0.000006 and 0.00005 for the time points 1 week, 6 months, 12 months and 24 months, respectively. Error bars show standard deviations.
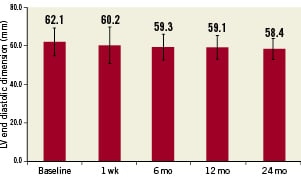
Figure 4. Average left ventricular end diastolic dimension showing a non-statistical trend towards improvement over baseline at all follow-up periods. Error bars show standard deviations.
Exercise time
The exercise tolerance test was performed at baseline and at 6, 12 and 24 months (Figure 5). All tests were stopped due to patient fatigue. No test was stopped for angina or other cardiac event. Mean values of exercise tolerance time increased from baseline of 246±113 sec to 303±141 sec (p=0.07) at 6 mo, 373±183 sec at 12 mo (p=0.007) and 371±181 sec at 24 mo (p=0.006).
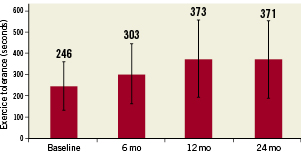
Figure 5. Average exercise tolerance time showing an increase that is not statistically significant at six months (p=0.07) but are significant at 12 months (p=0.006) and 24 months (p=0.007). Error bars show standard deviations.
Procedure time
Total average catheterisation time was 51±18 minutes with transendocardial cell injection time consuming 42±17.3 minutes. Figure 6 shows average time per injection presented as a function of sequential procedure number.
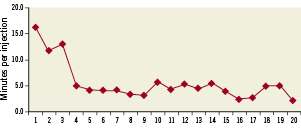
Figure 6. Average time per injection for each procedure presented sequentially by procedure number.
Discussion
The object of this open-labelled uncontrolled phase I study was to investigate the safety of percutaneously transplanting autologous bone marrow cells into myocardium using the helical needle transendocardial delivery system in stable coronary patients with ventricular dysfunction due to chronic myocardial infarction.
The present study uses one of the two catheter systems that is currently in clinical use for TIMD of cells. The helical fixation needle seems to enhance cellular retention by reducing back-leak into the ventricular chamber by the engagement of the helix, as well as by creating a longer self-sealing helical pathway into the tissue. Its safety and targeting accuracy has been shown in preclinical studies12,13,14.
Procedure safety is well-supported in this trial by the absence of treatment emergent major adverse cardiac events (MACE). This is noteworthy in that other recent cell therapy studies with the other TIMD catheter platform have experienced treatment emergent MACE including stroke15-17, cardiac perforation15,17,18, and ventricular arrhythmias17. These referenced studies, which do not all include results of these key safety parameters, have a treatment emergent MACE rate reported between 1.5% to 15% with the Myostar® catheter and endocardial NOGA electrical mapping system (Cordis, Irwindale, Diamond Bar, CA, USA) as compared to 0% reported here.
The safety profile observed in the TABMMI results with respect to stroke compared to the recent literature above, may relate to the shorter procedure duration facilitated by this fluoroscopically-guided catheter that does not require the additional time of endocardial mapping with NOGA. The absence of cardiac perforation in 170 TIMD performed in these 20 patients may result from: (1) the use of a helical needle whose sharp tip is at right angles to the catheter shaft as opposed to mounted along the catheter axis such as is the Myostar, (2) the needle being mounted on a very thin 5 Fr flexible coil shaft versus being mounted on a more rigid 8 Fr catheter shaft such as the Myostar, and/or (3) the use of a steerable guide catheter, which may reduce manipulation within the left ventricle. The two degrees of freedom of the Morph steerable guide catheter (deflection and rotation) are substantially the same as the Myostar; here we have the added Helical Infusion Catheter extension from within the steerable guide catheter, which enables reach to the ventricular wall for engagement. Although promising, the TABMMI procedure safety profile needs to be extended to other centres and additional patients, to determine if the data remains consistent over time. Although other catheter systems are being introduced today, it remains critical to individually consider the safety of each of them with respect to treatment emergent MACE.
This fluoroscopically-guided helical infusion system has today been shown to enable accuracy of delivery of 98.5% to target areas by an independent group14. Arguments in support of including NOGA mapping have been to provide precise delivery to the border zones characterised by NOGA. This remains a theoretical target without rigorous scientific support. Recent work by van der Vleuten 201019 has noted that correlation with infarct border zones on MRI and electromechanical endocardial mapping is limited although it states that it provides functional information on myocardial viability. Further, recent data presented has also shown that NOGA electrical maps are not well correlated with infarction on MRI20.
Penetration of the helical needle into the myocardium and deposition of cells in ten sites does however lead to a transitory elevation of both cardiac specific enzymes CKMB and troponin T11. This phenomenon has been observed in patients treated with straight needle TE catheter systems8,10. Neither CKMB nor troponin T levels correlated with the number of TIMD performed or with the duration of the procedure time. Its biologic significance and kinetics remain to be determined as it seemed to have no observable clinical effects.
The lack of sustained ventricular arrhythmia in any patient, and the total number of ventricular events recorded by 24-hour Holter were not significantly different compared to baseline data. This is in line with the growing body of preclinical and clinical work around TIMD of ABMMNC.
The safety of this treatment for the heart failure patient is also supported by the survival of 18 out of 20 patients treated for two and a half years. The first ten of these patients remain alive and well five years after the procedure. As shown by the Framingham study21-23, a mortality rate of 21% to 37% at one year, corresponding to four to seven patients out of twenty, would thus have been expected during the first year of follow-up in TABMMI from heart failure complications alone. However, only two patients died within the two-year follow-up reported: one at six months in an elective heart transplant surgery and one 23 months after the procedure, being therefore lost to follow-up.
The transthoracic echocardiographic ejection fraction measurements demonstrate improvement over baseline in this uncontrolled open-label safety study. These improvements in EF are not powered to confirm the clinical efficacy of this therapy but remain intriguing in a safety trial. Echo measurements of left ventricular end-diastolic dimensions also trended downward, suggesting overall anatomic and functional improvement despite the lack of statistical power.
Mechanism of action for the apparent beneficial effects shown here, and in studies with other TIMD catheters, remains a subject of continued exploration. The CD profile performed on the bone marrow cells here shows that an average of 1.87% cells are CD34 positive, corresponding to two million CD34+ cells for the dosage of 100 million ABM MNC cells. This number is on the same order of magnitude as a recently presented 167 patient phase II study focusing on these CD34 cells for the treatment of chronic myocardial ischaemia (CMI)18. As this CMI CD34+ study showed efficacy, it is possible that the therapeutic effect observed here may be related to this particular cell population. However, other cells present might also be beneficial; this requires further study. In this regard, clinical studies on pluripotent mesenchymal stem cells (MSC) derived from bone marrow in which mononuclear cells are cultured in vitro and then harvested for therapeutic administration through this same transendocardial delivery catheter, are now ongoing. The local delivery of the mononuclear cells does contain the source of these pluripotent MSC, and it is possible that the heart has potential to be an in vivo incubator enabling their potential benefit.
Potential advantages of TIMD over IC artery infusions include enhanced cell retention in target tissues, the avoidance of obstruction of the microcirculation, and the ability to target selected regions of interest directly, particularly those with poor perfusion. TIMD systems may also enable a more direct means for translation of basic research as most small animal studies use intramyocardial delivery to evaluate therapeutic strategies.
Perspectives
This study is limited in its design and scope from supporting any significant conclusions on the efficacy of the clinical approach reported. Although adverse events in four patients are unlikely to be due to the procedure itself, the absence of a control group prevents the elimination of this possibility. The results of this study have led to the initiation of a double-blinded randomised phase I/II study comparing autologous mononuclear bone marrow cells and mesenchymal stromal cells to placebo, as well as to a study comparing autologous MSC to allogeneic MSC derived from bone morrow. These two studies are actively recruiting in the United States.
Conclusions
This open-labelled uncontrolled safety trial of helical needle TIMD of autologous bone marrow mononuclear cells had no procedural complications or arrhythmias. The trend of all endpoints measured, including patient survival, supports the safety of the procedure and the technology involved. The data warrant additional larger trials to assess both the efficacy and comparative effectiveness of this approach in treating heart failure after myocardial infarction.
Acknowledgements
The authors would like to thank Dr. Licia Battaglia and Dr. Graciela Lucero for cell preparation; Dr. Jorge Miano, Dr. Cristian Bilos, Dr. Eduardo Barrera, Dr. Jose Ingols and Ms. Jessica Stertzer for clinical support; Dr. Cheryl Wong Po Foo for her assistance with the manuscript; and the entire support staff in Interventional Cardiology at the Instituto Argentino de Diagnostico y Tratamiento, Buenos Aires, Argentina.
Conflict of interest statement
BioCardia Inc., San Carlos, CA, USA, provided financial support for this study. P. Altman and D. Rouy are employees of BioCardia. A. Stertzer is a board member of BioCardia. The other authors have no conflicts of interest to declare.

