Abstract
Percutaneous coronary intervention and bypass grafting are effective for relieving symptoms and improving outcome in patients with coronary artery disease. Despite advances in medical treatment and revascularisation procedures, some patients with symptomatic ischaemic cardiomyopathy are not candidates for revascularisation. As life expectancy increases, interventional cardiologists and cardiac surgeons face patients with more complex disease, such as those with diffuse coronary disease that cannot be completely revascularised.
Introduction
End-stage coronary artery disease (CAD) is characterised by severe myocardial insufficiency, often with some degree of impaired ventricular function. Conditions resulting in no-option status include diffusely and small distal vessels, recurrent in-stent restenosis, chronic total occlusions, or comorbidities that preclude the use of conventional revascularisation techniques. Patients with no options may account for up to 12% of those referred for diagnostic catheterisation.1 In addition, an estimated 15% to 25% of patients undergoing coronary artery bypass graft (CABG) surgery will have one or more major target areas incompletely revascularised due to diffuse CAD.2,3
In this paper, we will discuss the alternative strategies that have been developed during the last few decades to treat symptomatic patients who do not have the option of standard therapies. We will focus on pre-clinical and clinical evidence of safety and the efficacy of stem cell therapy as a novel alternative treatment.
Alternative treatment in no-option patients
Non-conventional pharmacotherapy
A diverse group of drugs with anti-ischaemic effects may play a role in the alternative medical treatment of no-option patients.
METABOLIC MYOCARDIAL MODULATION
This term refers to a group of drugs (“partial fatty acid oxidation inhibitors”) that has no effect on blood pressure, heart rate, or left ventricular systolic function, but that inhibits fatty acid metabolism and promotes glycolysis, thus making the heart more energy efficient. This group comprises four different drugs: perhexiline, etomoxir, trimetazidine, and ranolazine, however, only trimetazidine and ranolazine have been widely studied in patients with chronic angina.
In a meta-analysis of 12 clinical trials in patients with stable angina, rimetazidine reduced the frequency of angina and increased the duration of treadmill exercise.4 Despite these encouraging findings, its potential in no-option patients has yet to be established.
Ranolazine, a new antianginal drug, has had anti-ischaemic effects in randomised trials (Table 1). Although its mechanism of action in angina remains unclear, ranolazine selectively inhibits late sodium influx and attenuates the abnormalities of ventricular repolarisation and contractility associated with ischaemia.5 In two randomised trials comparing ranolazine and placebo, or amlodipine in patients with chronic angina, patients on ranolazine have shown increased exercise duration and clinical improvement.6-8 Ranolazine has also been studied in patients with non-ST-elevation acute coronary syndrome in the MERLIN_TIMI 36 trial, in which 65,560 patients were randomised to receive either ranolazine or placebo. After almost 1-year follow-up, there were no significant differences in the primary endpoint (a composite of cardiovascular death, myocardial infarction, and recurrent angina). In addition, no statistically significant differences were noted in the secondary endpoints, which included all-cause death, hospital readmissions, and symptomatic documented arrhythmias.
The long-term safety of ranolazine was addressed in the ROLE (Ranolazine Open Label Experience) programme,9 which included patients who had completed the MARISA and CARISA trials and who were willing to participate in an open-label extension. The ROLE study comprised 746 patients who were being treated with ranolazine (between 500 to 2000 mg twice daily); the follow-up was extended up to 2.8 years. The most common symptom reported was dizziness (12%), followed by constipation (10%). Although QT interval prolongation of 2.4 ms was observed, no torsades de pointes were reported. Moreover, the survival analyses showed no increase in mortality in these patients. In conclusion, ranolazine is a safe, effective new drug for chronic stable angina; however, large-scale clinical trials are warranted to test various combination therapies.
POTASSIUM CHANNEL ACTIVATORS
Nicorandil is an arterial and venous dilator that improves coronary blood flow by opening the potassium channel, mimicking the natural process of ischaemic preconditioning. In comparison to placebo, nicorandil reduces death, nonfatal myocardial infarction, or unplanned hospitalisation in patients with chronic stable angina receiving other standard therapies. Recently, in a randomised trial comparing nicorandil and isosorbide mononitrate in patients with stable angina,10 nicorandil (5 mg) improved exercise capacity and prevented angina attacks. In addition, nicorandil was as equally effective as nitrates in improving the time to 1 mm ST-depression, total exercise time and time to onset of chest pain. In this trial, the only adverse reaction observed in the nicorandil group was headache. Despite this favourable safety profile, previous studies have associated nicorandil with ulceration throughout the gastrointestinal tract,11 however, the ulceration healed upon treatment withdrawal.
ANGIOTENSIN-CONVERTING ENZYME INHIBITORS
Angiotensin-converting enzyme (ACE) inhibitors increase coronary blood flow by reversing angiotensin II-mediated vasoconstriction. Two ACE inhibitors have been tested in patients with refractory angina: enalapril and ramipril. Enalapril reduced exercise-induced myocardial ischaemia in normotensive patients with normal left ventricular function and angina refractory to β-blockade.12 In the APRES trial,13 ramipril reduced cardiac death, acute myocardial infarction, and heart failure in patients with stable angina and left ventricular dysfunction who underwent revascularisation. Although no evidence is available in no-option patients, most of these patients are treated with ACE inhibitors because of previous infarction, left ventricular dysfunction, or hypertension or because they are at high risk.
IVABRADINE
Ivabradine specifically inhibits the If sinoatrial pacemaker current, thereby reducing the heart rate both at rest and during exercise without any negative inotropic action or unmasked alpha-adrenergic coronary vasoconstriction. Initial clinical data have become recently available. Borer et al14 demonstrated in a randomised study that ivabradine has beneficial effects on exercise tolerance in patients with stable angina, without a rebound effect after drug withdrawal. In addition, in two randomised trials, ivabradine showed comparable efficacy to amlodipine and atenolol in improving exercise tolerance.15,16 In the recently published ASSOCIATE study,17 patients with stable angina treated with atenolol were randomised to receive either ivabradine or placebo. At four months, patients treated with ivabradine showed a significant improvement in exercise capacity, and the drug was well tolerated. Therefore, ivabradine produced additional benefits in patients with persisting symptoms who were already being treated with atenolol. Although ivabradine may be an alternative treatment for no-option patients, its use requires further safety and efficacy assessments.
TESTOSTERONE
Intravenous19 and transdermal administration20 of testosterone has been shown to improve exercise-induced myocardial ischaemia in patients with stable angina, probably due to a coronary vasodilator effect and to increased blood haemoglobin levels. In 2008, Webb et al21 published a randomised study comparing testosterone with placebo in patients with CAD who had low plasma levels of testosterone. Although no differences were seen in global myocardial perfusion as measured by magnetic resonance imaging (MRI), the authors reported a significant improvement in perfusion only in the myocardium supplied by unobstructed coronary arteries. Testosterone may have potential benefits in patients with myocardial ischaemia and low levels of testosterone; however, this approach cannot be recommended in all no-option patients.
INTRAVENOUS THROMBOLYTIC THERAPY, ADENOSINE, AND HEPARIN
Intermittent administration of low-dose thrombolytic therapy dissolves thrombus and improves coronary blood flow at both the epicardial and myocardial levels. In two trials of urokinase in patients with refractory angina pectoris,22,23 exercise tolerance was increased as was time to ST-segment depression, without significant bleeding complications. However, both trials were limited by the small number of patients and the lack of a control group. Furthermore, urokinase is no longer available and other thrombolytic agents have not been studied for this purpose.
Adenosine may have a protective role, and heparin can accelerate the formation of coronary collaterals induced by ischaemia. Both drugs reduce the extent and severity of myocardial perfusion abnormalities in patients with refractory angina,24 but larger studies are needed.
Invasive strategies for treatment of no-option patients
TRANSMYOCARDIAL LASER REVASCULARISATION
Transmyocardial laser revascularisation (TMLR) is a technique that uses laser ablation to create transmural channels in the ischaemic myocardium in order to restore myocardial perfusion. The physiologic premise behind the application of TMLR is based on the work of investigators who were seeking to emulate reptilian circulation in the mammalian heart by creating conduits for blood flow from the ventricular cavity into the myocardium.
Although the mechanism of action is unknown, several theories have been postulated. In one theory, confirmed by a 123-labeled meta-oidobenzylguanide scintigraphic study,25 laser destruction of sympathetic nerve endings results in a form of cardiac denervation. Other theories include improvement in myocardial perfusion secondary to angiogenesis, or a placebo effect. Two types of laser have been used to treat refractory angina: the carbon dioxide (CO2) laser system and the holmium:yttrium-aluminium-garnet (Ho:YAG) laser system. In animal studies, histologic effects have been similar after six weeks, but increased thermoacoustic damage has been seen with the YAG laser.
Several randomised trials evaluating TMLR as a sole therapy for no-option patients have shown symptomatic improvement with the use of TMLR as compared with medical treatment. The first study, conducted by Schofield et al,26 involved 188 patients who were randomly assigned to either group. At one-year follow-up, improvement in angina scores and anti-anginal medications was observed in the TMLR group; however, exercise capacity did not improve. In a study by Frazier et al,27 192 patients were randomised to receive either TMLR or medical therapy. At 12-month follow-up, angina class, quality of life scores, and cardiac perfusion, as assessed by single-photon emission computed tomographic (SPECT) imaging, significantly improved. Similarly, Burkhoff et al28 reported an increase in total exercise tolerance and a better quality of life at one year, but they found no differences in myocardial perfusion or ejection fraction between the two groups. Aaberge et al29 described a significant reduction in angina symptoms and hospitalisations due to stable angina associated with TMLR, whereas left ventricular ejection fraction and mortality were seemingly unaffected. Interestingly, only one trial30 has demonstrated survival benefits in patients randomised to TMLR; however, no improvement in myocardial perfusion was observed between the groups. Finally, a meta-analysis of seven randomised trials involving 1,053 patients that evaluated the effect of TMLR showed a significant improvement in angina class but no improvement in survival.31 The effect on long-term survival is a key component to establishing the risk/benefit profile of any treatment. In the above-mentioned trials, complications after TMLR were mainly cardiac related and included myocardial infarction, left ventricular failure, atrial fibrillation, and ventricular arrhythmias. Perioperative mortality ranged from 3-5% in most reports, but rates as high as 12% have been described.
TMLR has been used as an adjunctive therapy to CABG surgery. The safety and efficacy of CABG surgery combined with TMLR versus CABG surgery alone have been assessed in only two randomised trials in patients in whom complete revascularisation was not possible. Allen et al32 randomised 263 patients to receive either combined therapy or CABG alone. At 1-year follow-up, survival benefits were observed in the combined group; however, the benefits were not associated with significant clinical improvement. In contrast, at 5-year follow-up, angina improvement was superior in the combined group, but no difference in survival was observed. Frazier et al33 randomised 49 patients and described a significant reduction in recurrent angina at 4-year follow-up.
Percutaneous TMLR has been suggested in order to reduce perioperative mortality associated with surgical TMLR. Results of randomised unblinded studies using percutaneous TMLR are similar to those seen with open-chest TMLR—symptomatic improvement without an increase in the survival rate34,35 or improvement in perfusion (thallium scintigraphy study) of the laser-treated regions.36 However, two randomised and blinded trials failed to demonstrate any favourable effect, emphasising the importance of the placebo effect.37,38
In conclusion, TMLR studies have shown clinical improvement without an increase in the survival rate. Moreover, assessing the placebo effect is difficult, as symptomatic benefits do not always correlate with objective findings (improvement in the left ventricular ejection fraction or myocardial perfusion).39,40 Therefore, TMLR should show a measurable physiologic benefit beyond the placebo effect before it can become an established therapeutic option for CAD.
ENHANCED EXTERNAL COUNTERPULSATION
Enhanced external counterpulsation (EECP) is a non-invasive procedure in which three sets of cuffs are used to compress the vascular beds of the leg and thigh in a sequential manner timed to the patient’s electrocardiogram. The cuffs are wrapped around the patient’s legs, and compressed air is used to apply sequential pressure (300 mmHg) from the lower legs to the lower and upper thighs during early diastole to propel blood back to the heart. Theoretically, this manoeuvre should result in a decrease in myocardial oxygen demand and an increase in coronary blood flow. This technique increases mean arterial blood pressure, retrograde aortic blood flow during diastole causing diastolic augmentation and increasing coronary perfusion along with venous return. In a multicentre, randomised controlled trial (MUST-EECP),41 139 patients with angina and documented ischaemia on treadmill exercise were randomised to receive 35 hours of active counterpulsation (300 mmHg of cuff pressure) or inactive counterpulsation (75 mmHg of cuff pressure) over a four- to seven-week period. The active counterpulsation group showed a significant decrease in angina episodes and nitroglycerin usage. Moreover, the time to ≥1 mm ST-segment depression increased in the active group as compared with the inactive group. Other registries with long-term follow-up that included more than 1,000 patients have shown similar results;42,43 EECP produced a sustained improvement in angina and quality of life. However, these clinical effects have not been associated with consistent improvement in myocardial perfusion.44
The mechanisms of the sustained antianginal effect of EECP are debated. It has been suggested that the increased exercised capacity after EECP therapy may be attributed, in part, to a training effect. Other beneficial effects associated with EECP include a decrease in circulating levels of proinflammatory biomarkers45 and an increase in the number and colony-forming capacity of circulating endothelial progenitor cells.46 Most of the experience with EECP comes from uncontrolled studies; therefore, in the current guidelines for treating patients with chronic stable angina, EECP is proposed as an alternative treatment for patients with no options for standard procedures with a IIB level of recommendation.47
NEUROSTIMULATION
Two types of neurostimulation are used to palliate angina by interrupting or modulating the afferent neural signals through which pain is perceived: transcutaneous electrical nerve stimulation (TENS) and spinal cord stimulation (SCS).
TENS is based on the “gate-control theory”: by stimulating large, non-nociceptive myelinated type A fibres transcutaneously at a high frequency, the system inhibits the impulse through smaller unmyelinated type C fibres, thereby reducing the activation of central pain receptors. In addition, TENS reduces sympathetic discharge, which leads to a decrease in cardiac work load and myocardial oxygen demand.48 In studies of TENS, patients have shown an increase in exercise capacity with a reduction of ischaemia noted on exercise electrocardiogram, a decrease in symptoms of angina, and a reduction in nitrate use.49 However, the development of skin irritations hampers the long-term use of the device.
In SCS, the epidural space is punctured at the level of the fourth or sixth thoracic vertebra, and an electrode is introduced in the T1 to T2 dorsal epidural space. An electrode stimulator is then placed subcutaneously in the upper left abdomen. Stimulation of these electrodes leads to a suppressed capacity of intrinsic cardiac sympathetic neurons to generate activity during myocardial ischaemia, thus decreasing pain sensation,50 and to a redistribution of myocardial blood flow from non-ischaemic to ischaemic areas.51 SCS does not appear to deprive the patient of a warning signal that leads to silent infarctions. Furthermore, SCS has anti-anginal and anti-ischaemic effects that seem to be secondary to a decrease in myocardial oxygen consumption. Three randomised trials comparing SCS with placebo have demonstrated a reduction in angina symptoms, an increase in exercise capacity, and a decrease in the degree of ST-segment depression at a given work load.52-55 (Table 2) The Electrical Stimulation versus Coronary Bypass Surgery (ESBY) trial56 showed that SCS and CABG surgery provide equal symptom relief. However, the CABG group had better exercise capacity and less ST-segment depression on maximum and comparable workloads. Long term follow-up has shown no differences in 5-year mortality; therefore, SCS may be an option for patients with severe angina who have a high surgical risk.57

In summary, symptoms and ischaemia seem to improve with either TENS or SCS, although the data supporting SCS are more convincing. However, neither procedure affects survival, myocardial infarction, the need for repeat revascularisation, or left ventricular function. Concerns associated with this approach include the invasive nature of SCS, the cutaneous side effects of TENS, and the presence of a strong placebo effect.
GENE THERAPY
Gene transfer technology with the use of growth factors has been proposed to treat refractory angina by inducing angiogenesis and arteriogenesis. Several growth factors stimulate angiogenesis and arteriogenesis: vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF) and platelet derived growth factor (PDGF); platelet activating factor (PAF); angioproteins; cytokines, such as interleukin(IL)-6 and IL-8; master switch genes, hypoxia-inducible factor-1 alpha; and nitric oxide. Nevertheless, the most effective and safest delivery strategy for inducing angiogenesis in ischaemic myocardium has not been determined.
VEGF increases the rate of endothelial cell proliferation, thus improving myocardial perfusion and angina functional class. VEGF has been used intramuscularly as VEGF-encoding plasmids and with adenoviral vectors. All patients treated with VEGF reported improvement in symptoms with no evidence of systemic or cardiac specific toxicity in Phase I trials58-60. The VIVA (Vascular endothelial growth factor in ischaemia for Vascular Angiogenesis) trial, a double-blind, placebo-controlled trial, evaluated the safety and efficacy of intracoronary and intravenous infusions of recombinant human vascular endothelial growth factor protein (rhVEGF). The study comprised 178 patients with refractory angina who were randomised to receive low-dose rhVEGF, high-dose rhVEGF, or placebo. At 120-days, angina improved significantly only in patients treated with high-dose rhVEGF. No differences were reported among the groups in exercise treadmill test or myocardial perfusion.
FGF increases the rate of endothelial cell proliferation. Patient perception of angina improved in preliminary studies in which basic FGF (bFGF) was administered in sustained-release microcapsules61 and recombinant FGF (rFGF), delivered by the intracoronary or intravenous route, was reported to improve SPECT perfusion abnormalities.62
In the first randomised, double-blind, placebo-controlled trial of gene therapy, recombinant adenovirus 5 FGF-4 (AGENT trial63 was delivered via the intracoronary route. Both the treatment and placebo groups showed improvement in exercise duration, thus demonstrating an important placebo effect. Only the subgroup of patients with exercise treadmill testing ≤ 10 minutes at baseline showed significant improvement in the treated group compared with the placebo group (1.6 vs 0.6 minutes, P=0.01, n=50). In the AGENT 2 trial64 52 patients with stable angina and reversible ischaemia were randomised to receive intracoronary injection of adenoviral particles containing a gene encoding fibroblast growth factor (Ad5FGF-4) or placebo. At eight weeks, Ad5FGF-4 injection had significantly reduced the size of the ischaemic defect (4.2% absolute, 21% relative; P <0.001), whereas placebo-treated patients showed no improvement (P=0.32). The AGENT 3 and 4 trials evaluated the efficacy of low- and high-dose treatment with Ad5FGF-4 in 532 patients with chronic angina in a randomised, double-blind, placebo-controlled fashion. Both studies were stopped because of a lack of efficacy after an interim analysis; however, a pooled data analysis from the two trials showed a significant improvement in total exercise treadmill testing time, time to 1 mm ST-segment depression, time to angina, and Canadian Cardiovascular Society class only in treated women, whereas there was no effect in men65, suggesting a gender-specific beneficial effect of gene therapy. The randomised FIRST trial66 evaluated the safety and efficacy of a single intracoronary infusion of recombinant FGF2 in 337 patients and found no improvement in exercise tolerance or myocardial perfusion. Although the use of gene therapy in patients with angina appears to be safe, efficacy data are controversial.
Another invasive technique that offers an alternative strategy for treating no-option patients is percutaneous in situ coronary venous arterialisation, but this approach is still in the experimental stage.
CELL THERAPY
Stem cell therapy has gained enormous interest since new insights have provided evidence that the heart may undergo a repair process in adulthood and that vasculogenesis may not be a paradigm found exclusively during embryonic development.67,68 By promoting angiogenesis, stem cell therapy has the potential to improve anginal symptoms in patients with chronic CAD.
Mechanism of neovascularisation
The creation of new blood vessels involves three different processes of supplying blood flow in ischaemic tissues: angiogenesis, arteriogenesis and vasculogenesis. Arteriogenesis is the process in which a pre-existing arteriole of the resistance vessel class matures into an artery of the conductance vessel class, whereas angiogenesis is the formation of new vessels by sprouting of endothelial cells from pre-existing vessel capillaries that originate from a pre-existing capillary. Vasculogenesis refers to the in situ differentiation of endothelial precursor cells to form capillaries. Previous studies have shown that some bone marrow-derived endothelial progenitor cells in the peripheral circulation are able to form new vessels in the heart. Ashara et al68 demonstrated that peripheral blood contains cells that can differentiate into endothelial cells in vitro and that endothelial progenitor cells are able to incorporate into sites of active angiogenesis in vivo. Using a canine bone marrow transplantation model in which the donor cells can be genetically recognised, Shi et al69 observed that cells from the transplanted bone marrow can be mobilised to the peripheral circulation and can form an endothelial layer on a previously implanted Dacron graft. Quaini et al70 used a cardiac chimerism model (female heart transplanted into a male host) to demonstrate that host cells can colonise the donor heart and can develop vessels.
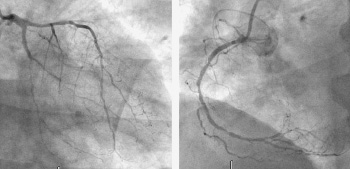
Figure 1. An example of a no-option patient. Left panel showing a severe and diffusely left anterior descending and distal circumflex and a sub-occluded marginal branch. Right panel diffuse disease in the right coronary artery
Preclinical studies using a chronic ischaemia model
Very promising results have been obtained with proof-of-concept, pre-clinical experiments using bone marrow stem cells. A wide array of data has been generated that support the use of stem cells to repair cardiac tissue in diverse clinical scenarios. Bhakta et al,71 using labelled cells and immunofluorescence microscopy, demonstrated that intracoronary delivered cells engrafted in the perivascular tissue. The safety of stem cell implantation has been shown in several studies. Li et al72 found no significant differences in systemic biochemistry in a chronic ischaemic model. Furthermore, Goodchild et al74 performed an electrophysiologic study seven weeks after stem cell treatment and reported no inducible ventricular arrhythmias in the treatment group, whereas arrhythmia was induced in one animal in the control group.
The efficacy of bone marrow-derived cells has been shown in several studies. Kinnaird74 showed that cell transplantation enhanced recovery of collateral flow in a murine model of hind-limb ischaemia; perfusion as measured by laser Doppler perfusion imaging was significantly higher in the treatment group than in the control group. Similarly, Fuch et al75 observed a significant improvement in myocardial perfusion in the ischaemic zone in the group treated with bone marrow-derived stem cells as compared with the control group in a pig model of chronic ischaemia. In addition, they observed an increase in myocardial contractility only in the treated pigs. Silva et al76 described a significant increase in vascular density and a significant decrease in myocardial fibrosis in animals treated with allogeneic mesenchymal stem cells as compared with control animals. Kawamoto et al77 demonstrated that NOGA- based transmyocardial injection of bone marrow-derived stem cell decreased the percentage of ischaemic area measure by NOGA and increased capillary density and left ventricular ejection fraction.
Clinical trials in no-option patients
Since 2003, several preliminary clinical studies have been performed to demonstrate the safety of bone marrow-derived stem cell implantation in no-option patients. Tse et al78 prospectively studied eight patients with stable angina refractory to maximal medical therapy who were treated with catheter-based intramyocardial bone marrow transplantation. At 3-month follow-up, symptoms improved as did myocardial perfusion and target wall motion as assessed by MRI. In the same year, Perin et al79 published a non-randomised, placebo-controlled study in which bone marrow cells were transplanted trans-endocardially in patients with severe heart failure due to ischaemic heart disease. The procedures were safe, and the authors reported a significant decrease in the reversible defect as measured with SPECT imaging and an improvement in myocardial volume oxygen consumption in the treated group as compared with the control group. Subsequently, three uncontrolled trials were published. Fuch et al80 transplanted bone marrow mononuclear cells via direct NOGA-guided injections in 10 no-option patients (Figure 2). The treatment was safe and decreased the ischaemic burden as shown on perfusion imaging. Briguori et al81 also treated 10 patients with NOGA-guided injections of bone marrow mononuclear cells and reported no cardiac events and clinical improvement at follow-up. In 20 patients, Beeres et al82 showed significant improvement in left ventricular ejection fraction from 51% to 54% when compared with baseline (P<0.01) and a reduction in end-systolic volume from 97±50 to 88±42 ml (P<0.01) as measured by MRI.
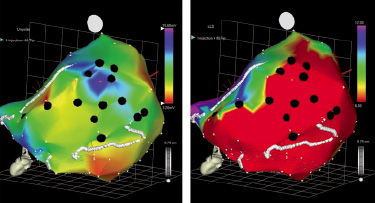
Figure 2. An example of a patient treated with stem cell with NOGA guided injections. Left panel univoltage map and right panel local shortening map. The black dots reflect the site of injections. In this particular case viable myocardium has been selected (high voltage and low local shortening)
The first randomised trial of a selected cell fraction derived from bone marrow was conducted by Losordo et al.83 In this trial, 24 patients were randomly assigned to receive NOGA-guided injections of an enriched fraction of CD34+ cells or placebo in a dose-escalating study. Although this pilot study demonstrated the safety of the procedure, it was not powered to assess efficacy. The preliminary results of the FOCUS trial, a randomised, controlled trial assessing the safety and the efficacy of bone marrow mononuclear cells in no-option patients, were recently presented by Perin et al (personal communication). At three months, they found significant improvement in both the Canadian Cardiovascular Society class and the percentage of reversible ischaemia as measured by SPECT imaging in the treatment group when compared with the control group. To date, stem cell studies have certainly only demonstrated the safety of this treatment in this clinical scenario; nevertheless, further clinical trials are warranted to establish the efficacy of this promising field.

