Introduction
The importance of revascularisation therapy on clinical outcome has been summarised in a recent meta-analysis of 28 randomised trials enrolling 13,121 patients1. Revascularisation by coronary artery bypass surgery (CABG) or percutaneous coronary intervention (PCI) in conjunction with medical therapy in patients with chronic stable coronary artery disease (CAD) was associated with significantly improved survival compared with medical therapy alone (OR 0.74; 95% CI, 0.63-0.88). This benefit was seen for both revascularisation modalities, CABG and PCI separately. Revascularisation was not associated with a significant reduction in non-fatal myocardial infarction compared with medical therapy (OR 0.91; 95% CI, 0.72-1.15).
Vascular restoration therapy (VRT) refers to a drug-eluting stent (DES) based percutaneous coronary revascularisation technology whose hallmark is the temporary nature of the biodegradable platform. As other percutaneous coronary revascularisation procedures, its primary goal is the relief of ischaemia by elimination of coronary luminal narrowing. The objective is achieved by temporary scaffolding of the arterial wall with concomitant release of antiproliferative agents. Both properties are required for a short period of time (3-6 months) following which biodegradation takes place with complete resolution of the device over the ensuing months and years. Assessment of endpoints must take into consideration the short- and long-term safety and efficacy of these complex implants.
Clinical trials evaluating the safety and effectiveness of this novel drug-device technology play an important role in it’s approval and adoption for clinical use. Although surrogate markers may have some role in the definition of device performance, direct measures of clinical outcomes are preferable in the understanding of the response of human subjects´ exposure to these products. Measures of success – or the other face of the coin – measures of failure, termed study endpoints, should serve several purposes as previously outlined in the Academic Research Consortium (ARC) Consensus Definitions for DES study endpoints2. They should reflect both short- and long-term pathophysiological properties of device action, they ought to represent clinically meaningful events, they must be clearly defined to ensure reproducibility in subsequent investigations, and they should be amenable to independent and blinded assessment procedures to ensure validity and avoid bias. The time interval of endpoint assessment following VRT deserves particular attention. Adverse events within 30 days are generally considered to be related to the device or the procedure. Conversely, competing sources become increasingly prevalent during longer-term follow-up. As a result the accurate distinction between device-related as opposed to underlying disease-related adverse events becomes increasingly difficult. There is consensus that despite of these limitations, follow-up after implantation of intracoronary devices and particularly VRT should extend to at least five years to capture rare and unanticipated adverse events.
Throughout this manuscript, we strongly adhere to the definitions of the ARC Consensus Document on Clinical End Points in Coronary Stent Trials2 and the Universal Definition of Myocardial Infarction proposed by the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction3. Since study endpoints should relate to the pathophysiological mechanisms most likely responsible for the clinical outcome, it is appropriate to identify the potential benefits and risks of VRT.
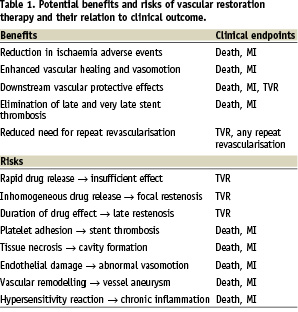
Potential benefits of VRT and relation to clinical outcome
VRT has the potential to go beyond the traditional properties of metallic coronary artery stents such as prevention of elastic recoil, treatment of dissections, and reduction of neointimal hyperplasia (particularly DES). Following complete biodegradation of the device, VRT leaves behind the healed arterial wall (in analogy to balloon angioplasty), which may result in beneficial vessel remodelling with plaque stabilisation and regression, and restoration of coronary vasomotion. If VRT proves to be successful, concerns regarding excessive stent length, multiple stent overlap, device fracture and anastomotic sites for subsequent CABG are all but eliminated. Moreover, VRT may revive the concept of plaque sealing by acting as a drug delivery vehicle to vulnerable plaque sites.
The phenomenon of very late stent thrombosis may no longer exist since the device has been completely absorbed and the vessel healed. As a corollary, prolonged dual antiplatelet therapy may no longer be necessary to protect against device-related long-term adverse events. Finally, bioabsorbable stents are compatible with MRI and multi-slice coronary CT imaging, enabling the non-invasive assessment of coronary artery patency following VRT.
Clinically, these potential benefits may translate into improved long-term outcome (Table 1). Although rare, stent thrombosis usually manifests itself by death or myocardial infarction, and there has been concern regarding very late stent thrombosis occurring steadily for several years after DES implantation4. Elimination of very late stent thrombosis would undoubtedly have a favourable impact and facilitate interdisciplinary decision-making in conditions requiring withdrawal of antiplatelet therapy to reduce bleeding complications such as in certain surgical procedures.
Coronary endothelial dysfunction has been associated with an increased risk for cardiac adverse events. Suwaidi and colleagues5 followed patients with mildly diseased coronary arteries who had undergone evaluation of coronary endothelial function by administration of intracoronary acetylcholine. Over an average 28-month follow-up (range, 11 to 52 months), patients with no or only mild endothelial dysfunction experienced no major cardiac adverse events. Conversely, patients with severe endothelial dysfunction suffered from numerous major cardiac adverse events including myocardial infarction, percutaneous or surgical coronary revascularisation, and/or cardiac death. Compared with bare-metal stents, drug-eluting stents have been shown to cause paradoxical vasoconstriction in the adjacent vessel segments suggesting device-induced endothelial dysfunction as the underlying mechanism6. More recently, a bioabsorbable everolimus-eluting coronary stent system showed restoration of coronary vasomotion at the treated as well as adjacent coronary artery segment at two year follow-up7.
Coronary artery stent fracture is associated with a high incidence of target lesion revascularisation. In a series of 307 lesions treated with sirolimus-eluting stents, the incidence of stent fractures is 2.6%, and most likely related to mechanical stress provoked by rigid structures and hinge points8. Although VRT may be limited by its physical properties as it relates to acute device performance (radial strength, device fracture), long-term adverse events such as stress-induced strut fracture may be of lesser concern.
Potential risks of VRT and its relation to clinical outcome
As any new technology, VRT may be subject to malfunction and failure, which owing to the critical localisation of the implanted device may place patients at risk for potentially serious adverse events. Disturbances in drug release kinetics may result in insufficient reduction of neointimal hyperplasia (restenosis), focal restenosis may be related to inhomogeneous drug distribution, and late restenosis may be caused by the long-term healing properties. Toxic drug effects could lead to tissue necrosis, cavitations or endothelial damage with impaired vasomotion. Failure of stent biodegradation would deprive patients from benefits of VRT and expose them to risks of conventional DES including very late stent thrombosis and abnormal vasomotion. Conversely, premature resolution of the stent platform may result in insufficient scaffolding and early restenosis. Hypersensitivity reactions to any of the stent components may result in chronic inflammation, pathological vascular remodelling, late stent malapposition, vessel wall aneurysms, and ultimately stent thrombosis.
The potential risks of VRT may manifest themselves clinically as death, myocardial infarction and the need for repeat revascularisation procedures (Table 1). It was recently shown, that very late DES thrombosis is associated with histopathological signs of inflammation and intravascular ultrasound evidence of vessel remodelling. Compared with other causes of myocardial infarction, eosinophilic infiltrates were more common in thrombi harvested from very late DES thrombosis, particularly in sirolimus-eluting stents, a finding, which correlated with the extent of stent malapposition9.
Non drug-eluting bioabsorbable magnesium stents, which degraded within four months in a first-in-man trial10, showed an ischaemia-driven target lesion revascularisation rate of 23.8% at four months, and an overall target lesion revascularisation rate of 45% after one year. By contrast, a bioabsorbable everolimus-eluting coronary stent system, which degrades more slowly, resulted in no need for target lesion revascularisation at one11 and 2-years7 follow-ups among 30 treated patients.
Assessment of device safety with VRT
Death
Death that occurs after a coronary stent procedure may by clearly related to a device- or procedure-related complication. Death may also occur unexpectedly during the follow-up period, either as a result of an evident cardiac event, unexplained sudden death or non-cardiac cause. The ARC2 consortium considered all-cause mortality as the least biased endpoint in a clinical trial or observational study. Owing to the fact that up to half of deaths may be non-cardiac in origin, the endpoint cardiac death affords more specificity when evaluating intracoronary devices. In order to avoid any underreporting, deaths should be considered cardiac unless an unequivocal non-cardiac cause can be established. Accordingly, cardiac deaths include all events related to a cardiac diagnosis, a complication of the procedure, treatment for a complication of the procedure, or an unexplained cause. We believe that especially for new devices and therapies such as VRT, cardiac death is an appropriate endpoint or part of a composite endpoint in a clinical trial.
Myocardial infarction
Myocardial infarction during a clinical trial of a percutaneous coronary intervention (PCI) device may occur during the immediate periprocedural period as a result of the index study procedure or long after the procedure. The latter may be related to spontaneous myocardial infarction owing to disease progression, subsequent revascularisation procedures of the target or non-target lesion, or result from late complications of the study device. According to the Universal Definition of Myocardial Infarction proposed by the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction3, myocardial infarction associated with PCI is classified as type 4a (periprocedural myocardial infarction after PCI), type 4b (myocardial infarction associated with stent thrombosis as documented by angiography or at autopsy) or types 1, 2 and 3 (spontaneous myocardial infarction – device unrelated).
PERIPROCEDURAL MYOCARDIAL INFARCTION
The occurrence of procedure-related cell necrosis can be detected by measurements of cardiac biomarkers before the procedure, and serial assessment at 6–12 hours and 18–24 hours. Elevations of biomarkers above the 99th percentile of the upper limit of normal after PCI, assuming a normal baseline troponin value, are indicative of post-procedural myocardial necrosis. Although there is no conclusive threshold for the diagnosis of periprocedural myocardial infarction, an elevation of CK-MB >3 ULN has been associated with increased long-term mortality. Pending further data and by convention, it is suggested in the aforementioned expert consensus document to designate increases more than three times the 99th percentile URL as PCI-related myocardial infarction. Although the measurement of troponin has been preferred over assessment of CK-MB in clinical practice, we recommend adhering to the historical definition of CK-MB exceeding three times the upper limit of normal to provide a well established historical framework for purposes of comparison.
Assessment of the quantity of myocardial damage (infarct size) may also be an important trial endpoint. Although the specific measurements vary depending on the assay and whether cardiac troponin T or I is used, in most studies troponin values correlate better with radionuclide-and MRI-determined infarct size than do CK and CK-MB. An analysis of 6,755 sirolimus-eluting stent treated patients demonstrated that irrespective of lesion complexity mortality at 6-month follow-up was highest among those who incurred a periprocedural myocardial infarction (4.3%), followed by those who developed any troponin elevation (2.5%) and significantly lower among those who remained free of these events (1.3%)12.
SPONTANEOUS MYOCARDIAL INFARCTION
Myocardial infarction after the periprocedural period may be secondary to late stent complications, repeat revascularisation procedures or progression of native coronary artery disease. Electrocardiography and angiography in selected cases may provide additional information to adjudicate the infarct site to either a target or non-target vessel. Spontaneous myocardial infarction includes type 1 (related to ischaemia due to a primary coronary event), type 2 (secondary to ischaemia due to increased oxygen demand or decreased supply), type 3 (sudden unexpected cardiac death, with evidence of ischaemia, but before confirmation by biomarker results), and type 4b (related to stent thrombosis as documented by angiography and/or autopsy). Myocardial infarction is diagnosed when blood levels of sensitive and specific biomarkers such as cardiac troponin or CK-MB are increased (any elevation above the upper limit of normal) in the clinical setting of acute myocardial ischaemia. Measurement of cardiac troponin is preferred over determination of CK-MB. Although elevations of these biomarkers reflect myocardial necrosis, they do not indicate it is the underlying mechanism (Table 1). Thus, an elevated value of cardiac troponin in the absence of clinical evidence of ischaemia should prompt a search for other aetiologies of myocardial necrosis.
The impact of spontaneous myocardial infarction on survival after PCI is worse than that of periprocedural myocardial infarction. Among 7,773 patients with acute coronary syndrome, the unadjusted mortality rate at one year was 16% for patients with spontaneous myocardial infarction compared with 6.0% for patients who had sustained a periprocedural myocardial infarction and 2.6% for those without myocardial infarction. After adjusting for baseline and procedural factors, spontaneous myocardial infarction remained a strong independent predictor of mortality (HR=7.49; 95% CI: 4.95 – 11.33, p<0.0001), while periprocedural myocardial infarction had no adverse effect on outcome (HR=1.30; 95% CI: 0.85-1.98, p=0.22)13.
Stent thrombosis after VRT
Stent thrombosis is a rare but devastating event, frequently associated with large myocardial infarction or even death. In bare-metal stent clinical trials of mostly non-complex lesions, stent thrombosis rates were approximately 1% with the use of dual antiplatelet therapy14, although higher rates (2% to 3%) were reported when more complex patients and lesions were treated15. Almost all events occurred within the first month and were not reported after 30 days by definition. In fact, it was not until late thrombosis events were recognised with increasing frequency during early brachytherapy clinical trials that reports of late thrombosis after bare-metal stents were recognised16. Initial reports of DES clinical trials showed no increased risk for stent thrombosis during the one year follow-up compared with bare-metal stents17, but concerns have been heightened by reports of increased risk beyond the recommended dual antiplatelet therapy period and continued risk beyond three years in real-world patients4. For definitions of timing and evidence of stent thrombosis the reader is advised to refer to the ARC Consensus Document2.
EARLY STENT THROMBOSIS
Although the aetiology of stent thrombosis is complex and multifactorial, early stent thrombosis is primarily related to procedural details and implantation techniques as well as sufficient inhibition of platelet aggregation rather than device-specific or patient-specific features. Therefore bioabsorbable stents are also subject to this type of complication. It may be assumed that prototype devices and initial operator experiences may be associated with a higher rate of early stent thrombotic events and other procedural complications owing to negligence of device-specific considerations. It has been recently shown that PCI centres with a high case load have a better safety record, resulting in lower 6-month rates of death and myocardial infarction compared with low volume institutions. Operator experience and device-specific techniques may be responsible for this difference.18
LATE STENT THROMBOSIS
An important potential of VRT may be related to the elimination of the disease entity of very late stent thrombosis. Of note, ischaemic events occurring beyond the designated time frame of degradation of the device can no longer be classified as stent thrombosis, except if stent struts or stent strut remnants are visualised at autopsy or by invasive imaging including angiography, IVUS or OCT. Documenting a thrombus at the original stent site by relying on permanent radio-opaque markers without evidence of stent remnants may not be sufficient to diagnose stent thrombosis. Therefore the categories probable and possible stent thrombosis will not be applicable to this technology. Ischaemia events occurring within the designated time frame should be classified according to the original ARC definition of stent thrombosis.
Assessment of device effectiveness with VRT
Prevention of restenosis and, therefore, repeat revascularisation will continue to be the measure of effectiveness in the VRT era. However, using the traditional measures of luminal restenosis may be hampered by the biodegradable nature of the stent device. Therefore, measures of effectiveness will differ according to the time interval from implantation, with traditional measures of restenosis used for permanent stent devices being applicable for the stented vessel/lesion, while alternative measures are required in the vessel after stent degradation. A separate chapter will discuss the applicability of late lumen loss in the VRT era. Target lesion revascularisation if regarded as a measure of device failure would not be reliable even in the presence of permanent radio-opaque markers, since a repeat intervention at the original stent site after its degradation may be due to restenosis but also due to disease progression. Therefore more global measures appear more appropriate – even if less specific – like target vessel revascularisation or even any repeat revascularisation procedure. These measures may also address potential positive device effects outside the treated lesions (down-stream effects).
Since VRT has the additional objective of restoring normal vascular function (vasomotion) beyond relieving luminal narrowing, some thought should be given to the assessment of this endpoint in clinical trials. Assessment of coronary vasomotion has the following limitations: at present there is no agreement on the optimal methodology for investigation of endothelial function. Rapid atrial or ventricular pacing, intracoronary acetylcholine infusion and dynamic bicycle exercise testing have all been used to quantify coronary vasomotion but lack standardised definitions. Moreover, baseline vasomotor tone and maximal vasodilatation may not be achieved in every case which may influence results. Lastly the reproducibility of endothelial function may be hindered by intra- and inter-individual differences as well as concomitant intake of vasoactive drugs. Therefore coronary vasomotion and assessment of endothelial dysfunction will be limited to selected patients and centres with extensive experience in the use of this technique.
Composite endpoints
As described in more detail in the ARC Consensus Document2, composite endpoints provide additional statistical power to detect meaningful differences between treatments; they may be either device-oriented or patient-oriented. As it relates to VRT, one would anticipate that the incidence of device-related complications will decline over time and be replaced by disease-related complications, the more global patient-oriented composite endpoint will become more relevant for follow-up of patients treated with such devices (Figure 1). The latter includes all deaths, any myocardial infarction, and any repeat revascularisation.
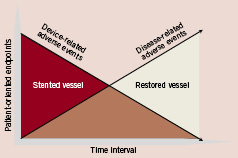
Figure 1. Clinical adverse events according to time course of vascular restoration therapy and relation to patient-oriented composite endpoints.
Clinical trials relevant to VRT
Clinical trials should be designed in a manner to investigate potential benefits of VRT compared to currently available alternative treatment strategies with careful assessment of adverse events. For stable coronary artery disease patients the following trials appear relevant. First, randomisation of VRT against established DES among more complex patients and lesions. Second, randomisation of VRT against medical treatment among patients with mild to moderate coronary artery disease. Third, randomisation against coronary artery bypass surgery for advanced coronary artery disease. For acute coronary syndrome patients, comparing VRT versus DES or bare-metal stents in culprit lesions, will be of interest particularly as it relates to long-term device-lesion interaction. Finally, the investigation of device performance and antiplatelet therapy in a 2 x 2 factorial design will address the important interrelationship of device therapy with antiplatelet regimens.
Conclusion
Vascular restoration therapy is a disruptive technology and requires a new framework in the assessment of device safety and effectiveness. Clinical trials designed to evaluate this novel technology must take into account the biodegradable stent nature and its designated time frame for setting the appropriate endpoints.

