
Abstract
Clinical heart failure prevention and contemporary therapy often involve breaking the vicious cycle of global haemodynamic consequences of myocardial decay. The lack of effective regenerative therapies results in a primary focus on preventing further deterioration of cardiac performance. The cellular transplantation hypothesis has been evaluated in many different preclinical models and a handful of important clinical trials. The primary expectation that cellular transplants will be embedded into failing myocardium and fuse with existing functioning cells appears unlikely. A multitude of cellular formulas, access routes and clinical surrogate endpoints for evaluation add to the complexity of cellular therapies. Several recent large clinical trials have provided insights into both the regenerative potential and clinical improvement from non-regenerative mechanisms. Initiating endogenous repair seems to be another meaningful alternative to recover structural integrity in myocardial injury. This option may be achieved using a transcoronary sinus catheter intervention, implying the understanding of basic principles in biology. With intermittent reduction of outflow in cardiac veins (PICSO), vascular cells appear to be activated and restart a programme similar to pathways in the developing heart. Structural regeneration may be possible without requiring exogenous agents, or a combination of both approaches may become clinical reality in the next decade.
Introduction
The quest for eternal youth by injection of liquids of animal organs as well as early work in xenotransplantation in the late 19th and early 20th century performed by Brown-Séquard and Voronoff are the first available sources of clinical attempts to regenerate tissues by transplanting organ extracts and later cells to enhance viability1,2.
However, the concept is in fact much older since it was first outlined and verbalised by the Swiss German medieval physician alchemist Philippus Theophrastus Aureolus Bombastus von Hohenheim, named Paracelsus, who wrote in his textbook “Die große Wundarzney” in 1534 that living tissues should be used to cure decay - “the heart heals the heart”. Although the present interpretation of this statement might be a stretch, it may be considered the first available description of the concept currently summarised as cellular regenerative therapies3. Transplantation of living cells was first performed by Paul Niehans, who can be regarded as the inventor of cell therapy and who treated celebrities such as Pope Pius XII and the German chancellor Adenauer. Later abandoned, evidence of the principal ideas remains visible in those performing “Frischzellentherapie” under obscure factual and legal conditions. Parabiosis is another construct that approaches the anti-ageing paradigm conceptually but is complex for consideration in clinical use4.
The unmet need for structural cardiac regeneration revived the idea of using cells to recover the heart.
The origin of cellular therapies in cardiac regeneration
FROM BONE MARROW TRANSPLANTS TO CARDIAC CELLULAR THERAPIES
Bone marrow was first given orally in early 1900 to treat various disorders, however unsuccessfully. In the late 1950s, Dr Donnall Thomas performed the first bone marrow transplant after radiotherapy in a patient with leukaemia in identical twins, and Georges Mathé in unrelated donors5.
Jean Dausset made a quantum leap in understanding genetically determined structures on the cell surface that regulate immunological reactions, the major histocompatibility complex, winning the Nobel Prize in Physiology or Medicine jointly with Baruj Benacerraf and George D. Snell in 1980. These observations prepared the ground for further discoveries in transplantation and regeneration research and facilitated concepts stimulated by the unmet need in cardiac recovery.
It now appears that paracrine effects inducing vasculogenesis and eventually cardiomyogenesis are the most likely avenue of regenerative pathways towards myocardial recovery. Previous theories were based on the proliferation of engrafted cells and integration into the syncytium of functioning myocardium. It became clear, however, that transplanted cells are lost, probably before showing any proliferative potential, but other mechanisms are more promising6,7 (Figure 1). Two mechanisms of action appear to play a role in the potential benefit of adult stem cells (mesenchymal stem cells, haematopoietic stem cells, endothelial precursor cells, cardiac stem cells). These include the paracrine action of cells or cellular components and the induction of endogenous repair.
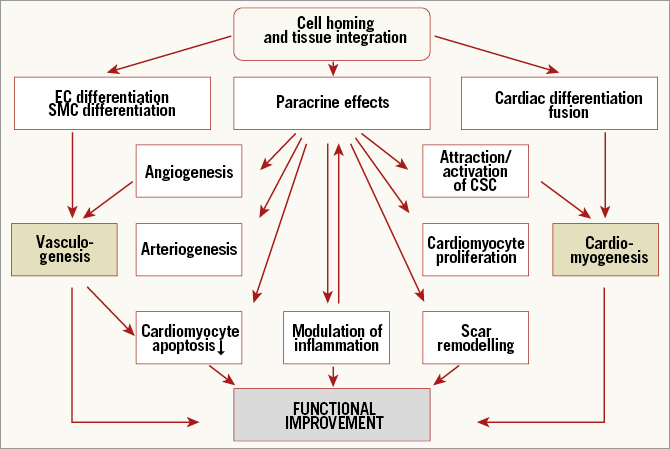
Figure 1. Current concept of cell homing and integration and potential effects of cellular therapies. Reproduced with permission from Dimmeler et al6.
A variety of novel approaches including patches with cells included in hydrogels and scaffolds, even 3D tissue constructs, and printed biomaterials is being evaluated to overcome the issue of cell survival and engraftment (Figure 2).
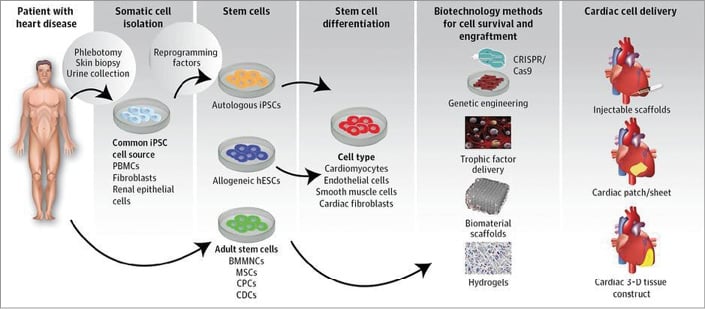
Figure 2. Next-generation cellular therapies. To overcome potential biohazards and to induce efficacy, several steps have to be undertaken to harvest cells and cellular factors, to expand the necessary regenerative signals and to incorporate them into effective cardiac cell delivery. Note that most of the harvesting tools as delivery routes require major interventions. Reproduced with permission from Nguyen et al10.
Recent reports on the efficacy of cellular therapies need to be interpreted cautiously in the context of the multitude of patient intrinsic and logistic variables, including cell delivery8.
Clearly, the target population itself is the most important factor. For patients with refractory angina and preserved left ventricular function or those with peripheral arterial disease, the challenge may be primarily to improve blood flow. For patients with recent acute myocardial infarction, the goal is to reduce scar size and remodelling. End-stage heart failure, the chronic expression of ischaemic heart disease, presents the largest unmet need and may be the most challenging patient population for cell therapies. There are potential differences in ischaemic and non-ischaemic cardiomyopathy with regard to the nature of myocardial involvement, and the goal of achieving reverse remodelling sets a high bar for therapies in these patients. Ischaemic cardiomyopathy itself is a heterogenous population including patients with predominantly myocardial scarring and those with hibernating myocardium.
THE COMPLEX NATURE RELATING CELLULAR THERAPIES TO CARDIAC RECOVERY
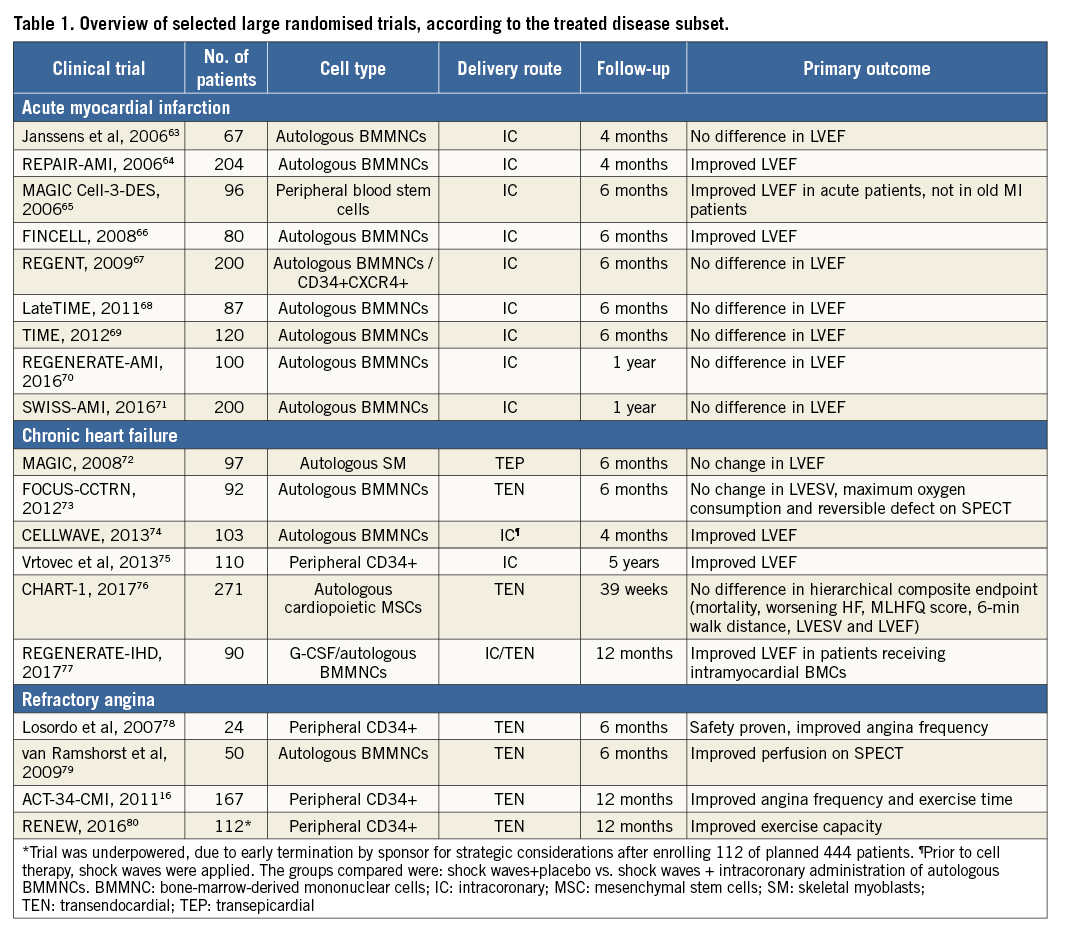
Recently, Nguyen published a systematic review on cellular approaches as well as potential strategies to overcome major clinical barriers9,10. For interventional cardiologists who rely on imaging and their dexterity in curing cardiac disease, it is difficult to dive into the complexity of basic and theoretical biology. Clinical surrogates of myocardial recovery and structural regeneration are multifaceted and rely on a variety of parameters. Some of them are still unrecognised. The simple causal relationship between a singular therapeutic impulse such as transplantation of cells into the adult and failing myocardium leading to improvement appears unlikely. Protagonists of cellular therapies admit that cellular expansion and survival are difficult to detect. Recently, stem cell tracking and multimodular imaging have paved the way not only to encounter survival of cells and add to efficacy monitoring, but also to augment safety, since risks of tumorigenicity and arrhythmogenicity may be detected, especially if larger patches impregnated with cells are used10. The use of reporter genes may help to elucidate cell fate beyond the scope of acute delivery and trafficking in vivo. Cells originally delivered into the myocardium may ultimately reside and exert a regional therapeutic paracrine effect in lymph nodes after cells traffic through myocardial lymphatics following delivery11. This requires a paradigm change from cellular differentiation, fusion and proliferation towards the broader hypothesis of induction of paracrine factors. Many questions remain: the key factors, the key pathways and the key influences of the jeopardised myocardium? Recently, the task force of the European Society of Cardiology regarding clinical trials on autologous adult stem cells in heart failure and acute myocardial ischaemia concluded that definite answers on the efficacy of cellular therapies are still unclear. For acute myocardial infarction, the phase III study BAMI (N=3,000) will provide insight into clinical endpoints. Enrolment is slow and it remains to be determined whether the statistical power for overall mortality and other clinical endpoints can be met12. Since the more important target in cardiovascular disease is the ability to impact on clinical outcomes in chronic heart failure, the results of the phase III DREAM-HF trial (N=600), including patients with both ischaemic and non-ischaemic cardiomyopathy, will be pivotal for the success of continued investigation in cardiac cell therapy13,14.
Another important parameter is healthcare assessment. A risk versus benefit ratio as well as a therapeutic burden for the individual patient represent important variables in the equation. Recently, at the SCAI 2017 meeting in New Orleans, Povsic from Duke University summarised the obvious benefits of CD34 transplantation in no option patients suffering from severe angina pectoris: “Patients receiving CD34+ treatment had more than a fourfold lower rate of mortality by 24 months (2.6% vs. 11.8%; p=0.003) and fewer instances of MACE (29.8% vs. 40.0%; p=0.08)”*. In spite of strong evidence for clinical improvements, including improvement in exercise tolerance and reduction in angina in patients with refractory symptoms, the only adequately powered phase 3 trial was halted prematurely for funding reasons15-17. The cost-effectiveness of harvesting, expanding and securing purity of cells depends on the size of the unmet need population. This illustrates the need for low-risk alternative therapies, showing equivalent clinical improvements.
Today’s perspectives in cellular therapies
In a provocative statement, Eugene Braunwald declared war on heart failure and not only summarised the rationale to battle disease, but also paved the way for alternatives of regenerative therapies18. He wrote, “several ethical regulatory legal and financial issues have to be addressed as biological therapies (gene therapy, cell therapy) progress to clinical application”.
These new trends require new less invasive and more cost-effective technologies. The reprogramming of adult autologous cells to induced pluripotent stem cells which then differentiate into the respective organotypic cell such as a cardiac progenitor cell is an example. Implantation of such progenitor cells may provide functional recovery of heart tissue. Translation from animal experiments to clinical trials will begin soon. These new technologies based on iPS cells represent a paradigm change in regeneration efforts. Patches, scaffold sheets and 3D constructs packed with genetically engineered cells as well as hydrogels and biomaterial scaffolds engrafted onto the surface of the heart are promising new formulas in cellular therapies.
To appreciate these new developments and translating concepts from cells to more complex alternatives, we discover new unexplored ground for interventional cardiologists, namely the Evo Devo Universe, described by the “EDU Scholarly Research Community” as an approach that “explores evolution, development and complexity augmenting theories by insights from information and computation studies, evolutionary developmental (evo-devo) biology, and hypotheses and models of quasi-evolutionary and quasi-developmental process applied at universal and subsystem scales”*.
This may be unfamiliar to practising physicians but, in the neo-Darwinian paradigm, adaptive evolutionary development guides the production of ordered, complex and intelligent structures, which on the other hand is the trajectory of the paradigm change needed in regeneration research. Therefore, this elementary principle of cardiac rejuvenation should be called “Devo Re Universe” (development regeneration), based on previous studies collecting the similarity of signalling pathways in cardiac development and regeneration19,20. Combining regeneration research with principles from cardiac development might be one of the alternatives for future research, and several authors have discussed the potential of this concept of using developmental signals to induce regeneration21. As depicted in Figure 3, the nature of the signal restarting regenerative pathways is undefined and may be the embedment of cells, a recruiting signal for resident stem cells or, as we describe in our hypothesis, the restart of an environmental signal such as an activation of vascular cells by blood flow22. This undetermined signal has to coincide with the second signal of myocardial injury.
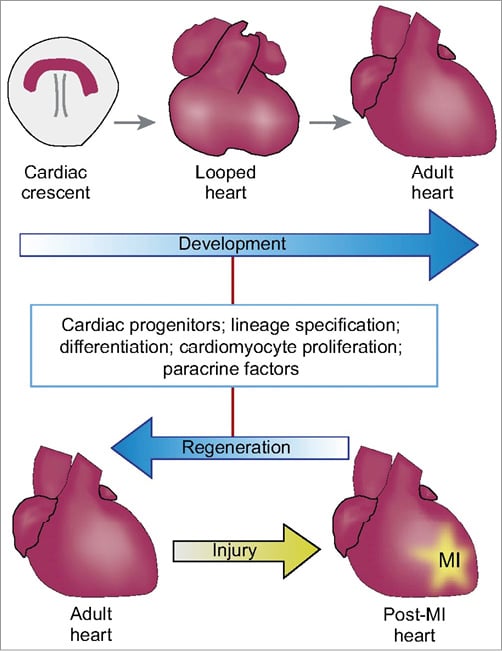
Figure 3. In jeopardised hearts regeneration may occur using developmental signals. Reproduced with permission from Christoffels et al21.
The concept of stimulating endogenous cardiac repair
The basic idea to use signalling in endogenous repair without transplanting cells is to initiate innate pathways to establish regeneration. Recovery in structural integrity after an acute or chronic cardiac event requires a synchronicity of the regenerative potential and homeostasis among the three structural components - cardiomyocytes, vascular cells and extracellular matrix. Whereas scarring and remodelling after myocardial injury is the norm, it is detrimental for the patient. Therefore, we have to evaluate the library of canonical developmental pathways to consider new approaches to heart failure therapy.
Mammalian hearts are complex in development, structure and function. From single heart tube spatiotemporal gene expression patterns, cytoplasmatic gradients, signalling of various origin, cellular migration, transformations and even epigenetic influences such as local haemodynamics contribute to the ultimate heart design. The assignment of circulatory demand in the developing heart requires a sharing of cellular functions assembling into tissue-generating cohorts. In adult hearts, syncytial cardiomyocytes slide along fibrous septations, electrical signals travel in preformed specialised cells, and valves close and open ejecting blood according to contractile forces and circulatory demand. It is therefore not surprising that this unique organ needs cellular homeostasis to guarantee long-lasting adaptive function. However, a consequence of this “complex” architecture and functional ability is that a “scarless” regenerative potential present in more “primitive” species is lost or at least dormant and disabled in adult mammalian hearts23,24. Scarless recovery is lost probably because the complex nature of the pathophysiology of mammalian myocardium during ischaemia/reperfusion allows only asynchronous triggered repair mechanisms and because of the energy loss in deprived myocardial zones. What is little appreciated is the fact that, even in this tight structural straightjacket, 50% of cardiomyocytes are renewed during the lifespan of individual humans, showing that the basic principles of regeneration per se persist in adult hearts25,26. It is very likely that paracrine signals originating from the “non-cardiomyocytes” and the extracellular matrix might be the “controller” of coordinated repair27-29. Even arrested developmental pathways, such as the Hippo signalling controlling organ size in animals through the regulation of cell proliferation and apoptosis or the fact that cardiomyocyte proliferation is by and large aborted in newborn rats, illustrate that “myocardial growth signals” are present in mammals, though actively suppressed for the greater good of organ homeostasis30. Indeed, cardiomyocyte regeneration is observed in a murine model of MI when the Hippo signalling pathway is knocked out genetically or YAP is overexpressed. The clinical exploration of such an approach has recently been proposed31. The overriding question, however, remains how to operationalise the facilitation of these conserved mechanisms in injured myocardium.
Several concepts, spanning genetic engineering, cellular reprogramming and stem cell transplantation and even the application of pharmaceutical agents such as beta-blockers, statins and ACE inhibitors, have been tried and are under investigation in randomised trials12,32,33.
Alternatively, another important regenerative principle has been appreciated in the past. Is a re-activation of imprinted developmental processes per se able to restart programmes able to recover the dysfunctional heart34?
Today’s insight into the complex nature of endogenous repair can only be explained in part and shares the similar “broad explanation” of paracrine activation stated by classic cellular therapies. Therefore, we should concentrate more on the targets of structural repair and recovery. As is known from in vitro cardiomyocyte cell index studies with the xCELLigence system, that allows quantification of cellular proliferation via changes in electrical impedence enacted by cellular growth and proliferation, the detection of enhanced proliferation does not discriminate on cellular sources including ckit cells, but rather on the structural repair embedding resident mesenchymal stem cells (cardiomyofibroblast cells), activated progenitors or dividing adult and failing cardiomyocytes.
THE DOMINO EFFECT OF RESTARTING DEVELOPMENTAL PROCESSES
Reviving developmental pathways by a restart of a molecular signalling event might be the purest form of endogenous repair. We hypothesised this signalling event to be an influence of haemodynamics on a target cell line, which originated from endothelium experiencing the same impulse in embryonic life22,34,35. Although unproven, re-entry into canonical developmental pathways in the adult failing heart may eventually lead to some form of healing process, otherwise unused. In combination with the first important stimulus “myocardial injury”, a restart and impulse of pleiotropic signals leading to neoangiogenesis, remuscularisation and matrix organisation in a controlled fashion, as during embryogenesis, is an attractive idea. To prove this hypothesis, one has to find the same signals in the embryo to provoke these signals in the adult failing heart. Potential attractive candidates for this signalling event might be non-coding RNA since they link epigenetic signals to gene expression patterns, are known to play an important role in vascular biology, and are conserved in evolution, meaning that many species are dependent on their action36.
HAEMODYNAMIC FORCE SCULPTURING THE DEVELOPING HEART
Influencing cardiac endothelium-myocyte interaction has been identified as a clinical opportunity for new heart failure therapies34,37. The role of mechanotransduction and the sculpturing force of embryonic haemodynamics have been recognised for a long time and are regaining scientific focus reconnecting the “mechanistic” stimulus to molecular cascades38-40. On day 21/22 the human heart starts beating. This primordial heartbeat has to be sensed regulating subsequent cardiogenesis. As in zebrafish, non-coding RNA seem to play an important role in signal transduction connecting mechanotransduction to phenotype development. Heartbeat initiates miR-143 expression, which is an essential component of the mechanotransduction cascade. Knocking down miR-143 results in de-repression of retinoic acid signalling, and produces abnormalities in the outflow tracts and ventricles. Key regulators of normal cardiac development and deviations such as congenital heart disease have been identified in the past, but the complexity of the underlying principles has not yet been deciphered41.
In our own work on the similarity of structural malformations of inherited and sporadic atrial septal defects, we hypothesised that a tissue-generating cycle involves identifying the gain of morphogenetic function in special regions of the developing heart as well as loss of function as key elements in cardiogenesis42. One example would be contraction patterns in the looping heart with an increase of shear stress and pulsatile stretch locally, whereas flow distribution would be reduced in other areas leading to apoptosis. The local miRNA expression as a consequence of mechanotransduction in the primitive embryonic heart seems to be this predicted gain of function sensing as an epimorphic sculpturing event promoting further development43,44.
THE TERM “EMBRYONIC RECALL”
The central idea of the “embryonic recall” hypothesis is that this gain of morphogenetic function sensing is a process that can be revived in the adult failing heart by reiterating the same mechanism on cellular descendants involved in primary sensing. Recently, Darren Gilmour et al underscored this feedback loop coordinating tissue shaping by mechanical cross-talk45. They concluded that “mechanical forces that drive tissue-shaping processes provide another important mechanism for feedback between cell form and fate”. This is exactly the central idea of our hypothesis “embryonic recall”. Unlike the present concepts of regeneration, this involves an epigenetic phenomenon, a process rather than cellular approaches. In this hypothesis, we reiterate the gain of morphogenetic function sensing by a temporary elevation of pressures in cardiac veins. Hierck et al demonstrated molecular signalling by primary cilia of endocardial cells and the relationship to morphogenesis of the heart46. Similarly, blood flow changes within cardiac veins may be sensed by the same mechanism and, according to our hypothesis, restart developmental pathways (Figure 4).
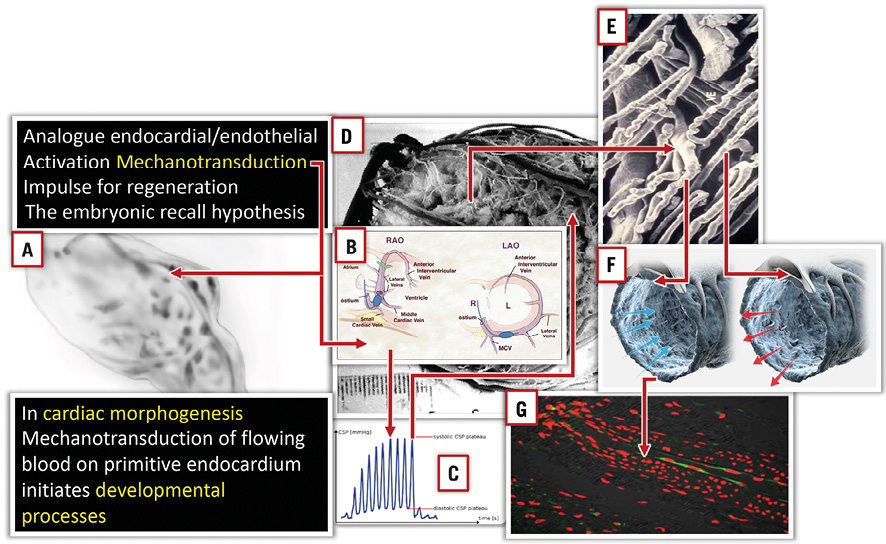
Figure 4. Mechanical forces influencing cardiac development via mechanotransduction of shear stress and pulsatile stretch. The same situation can be expected in the venous microcirculation during brief periods of pressure increase and flow reversal. A) Primitive heart tube before looping. B) & C) PICSO brief pressure increase in the coronary sinus reverses flow into the microcirculation (D, E, & F). Arrows in panel F indicate force vectors and reversal of flow; note bended primary cilia according to flow direction. This mechanism is thought to be the central impulse to restart developmental signalling according to the embryonic recall hypothesis.
This is in line with the above-mentioned theory of development/regeneration (DEVO/REG), meaning that a singular impulse may be able to restart the next level of morphogenetic pathways. It is our claim that environmental changes (i.e., pressure increase in cardiac veins and activation of endothelial mechanotransduction) are sensed as a similar signal from the failing tissue just as the developing tissue reacted to an analogous signal (as in our case shear stress of flowing blood and induction of growth signals in endocardial cells).
A transcoronary sinus catheter intervention might be a practical way to prove this hypothesis in humans47.
Cardiac veins have primary cilia within their lumen and elevating retrograde blood flow intermittently creates a mechanotransduction effect, thereby restarting developmental pathways48. Next to the known effects on myocardial salvage and clearing of the obstructed microcirculation, this may induce structural regeneration. Evidence from a preliminary clinical trial suggests the same signalling pathway using microRNA as found during development as well as other cytoprotective, proliferative and vasoactive molecules49,50.
Pressure-controlled intermittent coronary sinus occlusion (PICSO) is a transcoronary sinus catheter intervention temporarily elevating pressures in cardiac veins, which has been proven in myocardial ischaemia/reperfusion syndromes51,52. The dual principles of PICSO are, first, redistribution of flow towards deprived myocardial zones with subsequent clearance of myocardial obstruction, and second, the re-entry into morphogenetic molecular pathways via mechanotransduction and activation of venous vascular cells. Basic principles of PICSO in the context of endogenous repair are depicted in Figure 5 and Figure 6, showing the available catheter and control system for temporarily elevating the pressure in cardiac veins. Reflow into the venous microcirculation and the abrupt change in flow direction are believed to induce mechanotransduction and activation of vascular cells. Pressure monitoring and feedback control are necessary to avoid interference with coronary artery inflow. Long-term data on myocardial salvage of this intervention support the underlying theory of endogenous repair and potential clinical benefits52,53.
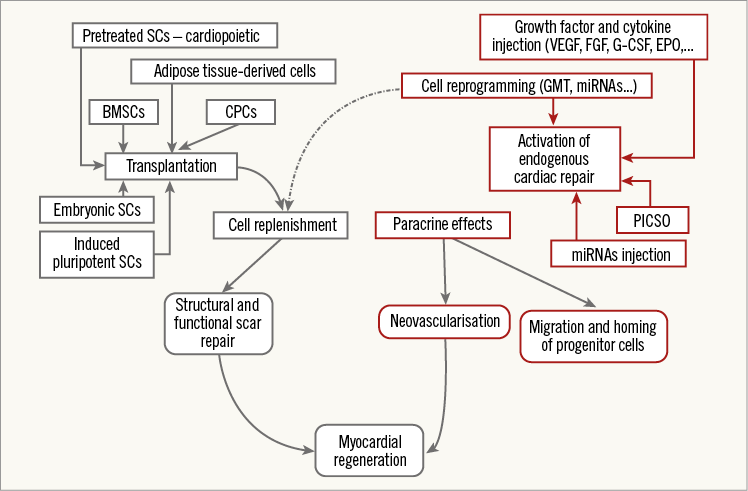
Figure 5. PICSO in the context of other methods in endogenous repair. Transplantation of stem cells vs. initiation of endogenous myocardial regeneration without cell transplantation. Depicted are two basic approaches to myocardial regeneration: (1) via transplantation of different types of stem cells into the damaged myocardium (on the left, in blue); and (2) by activation of endogenous pathways of cardiac repair, without cell transplantation (on the right, in red). Both sets of techniques are thought ultimately to exert their effects on the paracrine route and by replenishing lost cardiomyocytes. BMSCs: bone marrow-derived stem cells; CPCs: cardiac progenitor cells; EPO: erythropoietin; FGF: fibroblast growth factor; G-CSF: granulocyte colony stimulating factor; GMT: Gata4, Mef2c, Tbx5; miRNAs: microRNAs; PICSO: pressure-controlled intermittent coronary sinus occlusion; SCs: stem cells; VEGF: vascular endothelial growth factor. Reproduced with permission from Milasinovic et al81.
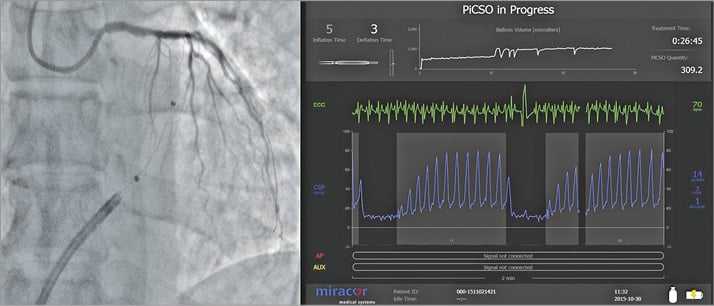
Figure 6. Transcoronary coronary sinus catheter intervention (PICSO). Catheter in the coronary sinus during balloon inflation. Coronary sinus pressure during PICSO. This catheter intervention may be effective as regenerative therapy by activating vascular cells in cardiac veins.
Figure courtesy of Miracor Medical Systems GmbH, Vienna, Austria.
Conclusion
State-of-the-art cellular therapies remain a promising tool in regaining cardiac recovery. Emerging new concepts are currently being tested, introducing a broader perspective. Basic principles of recovery and targets of cardiac regeneration warrant new insights into processes regaining structural integrity. Translating cellular transplantation with its current mode of action of paracrine effects into codes of endogenous repair opens up a new horizon. During development, similar principles are triggering cardiac growth as can be reactivated during myocardial jeopardy. The theory of “embryonic recall” claims that reactivation might occur using mechanotransduction on venous vascular cells. This activation can be achieved with a transcoronary sinus catheter intervention (PICSO). Although the complex nature of this initiation and re-entry into developmental cascades to achieve structural recovery is currently not well understood, preliminary results seem to underscore the claims of the underlying theory.
To explore fully the potential of endogenous repair using transcoronary sinus catheter interventions, new clinical trials are needed, in tandem with basic research to decipher the options of using developmental signals for regeneration of the heart.
Authors’ perspectives
In the future, we have to diversify our research on cardiac regeneration. We need to design adequately powered clinical trials which advance the field. As of May 2017, 405 interventional cardiovascular cell therapy trials are registered in clinicaltrials.gov as recruiting, and an additional 76 are to begin. In contrast, 966 studies are registered as complete, 187 were terminated due to various reasons, 19 studies were suspended and 51 trials have been withdrawn. Comprehensive meta-analyses suggest the vast benefit in patients with refractory angina15-17, with more modest and conflicting results in patients with acute myocardial infarction54,55 or chronic heart failure56-58 (Table 1). To understand fully the risks and benefits of cellular therapies, we await the results of large ongoing phase III clinical trials, including BAMI and DREAM-HF. However, there are new modalities, agents and technologies such as exosomes, MRNA, patches with new forms of cell types, delivery methods and formulas that merit further exploration33,59. We have to look for therapeutic alternatives for refractory angina, acute myocardial infarction and chronic heart failure. Induced pluripotent cells differentiated into cardiac progenitors capable of forming functionally active adult cardiac cells appear promising in preclinical studies and will be translated into clinical trials soon60. If we consider and re-interpret concepts that are co-evolutionary available to cellular approaches, such as endogenous repair, including recent findings on therapeutic interference with genetic pathways responsible for structural cardiac recovery61,62, and findings nurtured by developmental and system biology as claimed by our hypothesis “embryonic recall”, we may find that several options of initiation of endogenous repair are already in existence, potentially complementing cellular therapies.
For future regeneration research, we have to make sidesteps into the developmental biology of the heart and enhance the current options and alternatives to cellular approaches.
Cell therapy research has created a new dimension in cooperation between basic scientists and interventional cardiologists. Biotechnology and medical technology have assisted in the development of clinical applications, and research will prosper as long as the industry shows sufficient interest. Challenges remain, and successful approaches may be discontinued in spite of clearly positive results in clinical trials. On the other hand, we need to evaluate the risk benefit ratio for the individual patient and find straightforward solutions in the future.
CROSSROADS
We need to understand the nuances between different patient populations (for example, refractory angina vs. acute myocardial infarction) and design strategies to meet their needs. This may include cellular therapies or potential modifications (exosomes), but we also need actively to investigate ways to enhance endogenous repair.
The entry into canonical developmental pathways seems promising. This may induce the domino effect necessary to regain structural integrity of injured myocardium. Several options are available, including the induction of a dormant developmental process as proposed in the theory of “embryonic recall”. Although this theory seems complicated and needs to be fully developed, it may be easy to perform and test clinically.
Developmental pathways within a failing adult heart are different from the morphogenetic mechanisms in the developing heart. Although it seems to be attractive that parameters in the signalling cascade may induce an ongoing domino effect, signals can only be estimated from analogy to the developing heart. Many of the signals that directly influence myocardial structure or secreted morphogens may have differing effects according to the underlying milieu. In turn, the paracrine factors thought responsible for the effects of cell therapy today must be more precisely defined.
Ultimately, all regenerative options need to be tested in large randomised clinical trials. The patient with the unmet need should be our primary concern. The application of interventions will be directly influenced by the perceived accessibility and patient safety in clinical trials. This may favour the mechanotransduction concept, using haemodynamic force to activate vascular cells entering developmental pathways. The PICSO concept is already used clinically and we can expect to reconnect molecular events to clinical outcomes. To test this hypothesis, we are currently planning a clinical trial in heart failure patients, which will disprove or support our hypothesis and will give insight into whether the theoretical concept can connect to clinical significance.
Conflict of interest statement
W. Mohl has received funding for PICSO studies from the ATOS I Austrian Science Fund (P13274-Med), the Austrian Federal Ministry of Traffic, Innovation and Technology, Vienna, Austria (BMVIT GZ609.637/000-III/12/2004), and the International Society of Coronary Sinus Interventions (ISCSI), and is a founder of Miracor and the Angel Valve Project. T. Henry has received research funding from NIH, Vericel, Mesoblast and Capricor. E. Perin has received research funding from NIH, and Mesoblast. The other authors have no conflicts of interest to declare.

