
Abstract
Aims: The DYNAMIC trial assessed the safety and explored the efficacy of multivessel intracoronary infusion of allogeneic cardiosphere-derived cells (CDCs) in patients with heart failure and reduced ejection fraction (HFrEF). Here we report the results of the DYNAMIC trial.
Methods and results: We enrolled 14 patients with EF ≤35% and NYHA Class III-IV despite maximal medical and device-based therapy in this single-centre, open-label trial. Intracoronary catheterisation delivered four escalating doses (totalling 37.5-75 million cells) by sequential non-occlusive technique to all three major coronary arteries. The primary safety endpoint was a composite of post-infusion TIMI flow, ventricular tachycardia/fibrillation, sudden death, major adverse cardiac events or acute myocarditis within 72 hours. Twelve patients were male and EF averaged 23.0% (±4.5%). No primary safety endpoints were observed. Two patients died of HFrEF progression nine and 12 months following infusion. Compared to baseline, there was an improvement in EF (26.8% vs 22.9%, p=0.023) and left ventricular end-systolic volume (139.5 vs 177.8 cm3, p=0.03) at six months. Quality of life (QoL) scores and NYHA class (p=0.006) improved at six months. At 12 months, the improvement in EF and QoL remained significant.
Conclusions: Global intracoronary infusion of allogeneic CDCs is safe and feasible. The efficacy of allogeneic CDCs in HFrEF needs to be tested in larger randomised trials.
Introduction
Intracoronary administration of autologous cardiosphere-derived cells (CDCs) post myocardial infarction (MI) is safe and results in decreased scar size, increased viable myocardium and improved regional function of infarcted myocardium one year post treatment1,2. While the initial clinical studies with CDCs were focused on autologous therapy, preclinical studies revealed that allogeneic CDC administration without immunosuppression was safe, promoted cardiac regeneration, and improved heart function, with efficacy comparable to autologous CDCs3,4,5. Experiments in small and large animal models of ischaemic and non-ischaemic cardiomyopathy have demonstrated that exogenously administered CDCs act by transiently stimulating endogenous reparative and regenerative pathways (rather than through direct differentiation into newly formed myocardium)6,7,8,9,10. This indirect mechanism of action rationalises the efficacy of allogeneic cells, which have been shown to persist in the host myocardium of experimental animals long enough to initiate the same cascade of indirect effects as autologous CDCs, thereby conferring indistinguishable benefits3,5. The comparable effects of allogeneic versus autologous CDC therapy led to three clinical trials evaluating the safety and efficacy of allogeneic CDC therapy: the Dilated cardiomYopathy iNtervention With Allogeneic MyocardIally-regenerative Cells (DYNAMIC) trial (NCT02293603, reported here); the ALLogeneic heart STem Cells to Achieve myocardial Regeneration (ALLSTAR) trial (NCT01458405) in patients post MI11; and the Halt cardiOmyopathy ProgrEssion (HOPE)-Duchenne trial (NCT02485938) in patients with Duchenne muscular dystrophy. Here we report the results of the DYNAMIC trial, a single-centre, prospective, dose-escalation study of non-occlusive sequential triple-vessel intracoronary infusion of allogeneic CDCs in patients with heart failure with reduced ejection fraction (HFrEF).
Methods
TRIAL DESIGN
The study protocol (Figure 1; complete protocol in Supplementary Appendix 1) was approved by the institutional review board of Cedars-Sinai Medical Center. All participants provided written informed consent. DYNAMIC enrolled 14 subjects with HFrEF (EF ≤35% as determined by a transthoracic echocardiogram [TTE] within the preceding six months) and EF ≤40% on screening cardiac computed tomogram (CT) and New York Heart Association (NYHA) Class III or ambulatory Class IV heart failure, despite guideline-directed medical and device therapy for at least three months. Subjects meeting all inclusion and no exclusion criteria were enrolled. Although it was not mandated by the study protocol, all patients underwent coronary angiography prior to enrolment to confirm that there was no need for revascularisation prior to enrolment in the study.
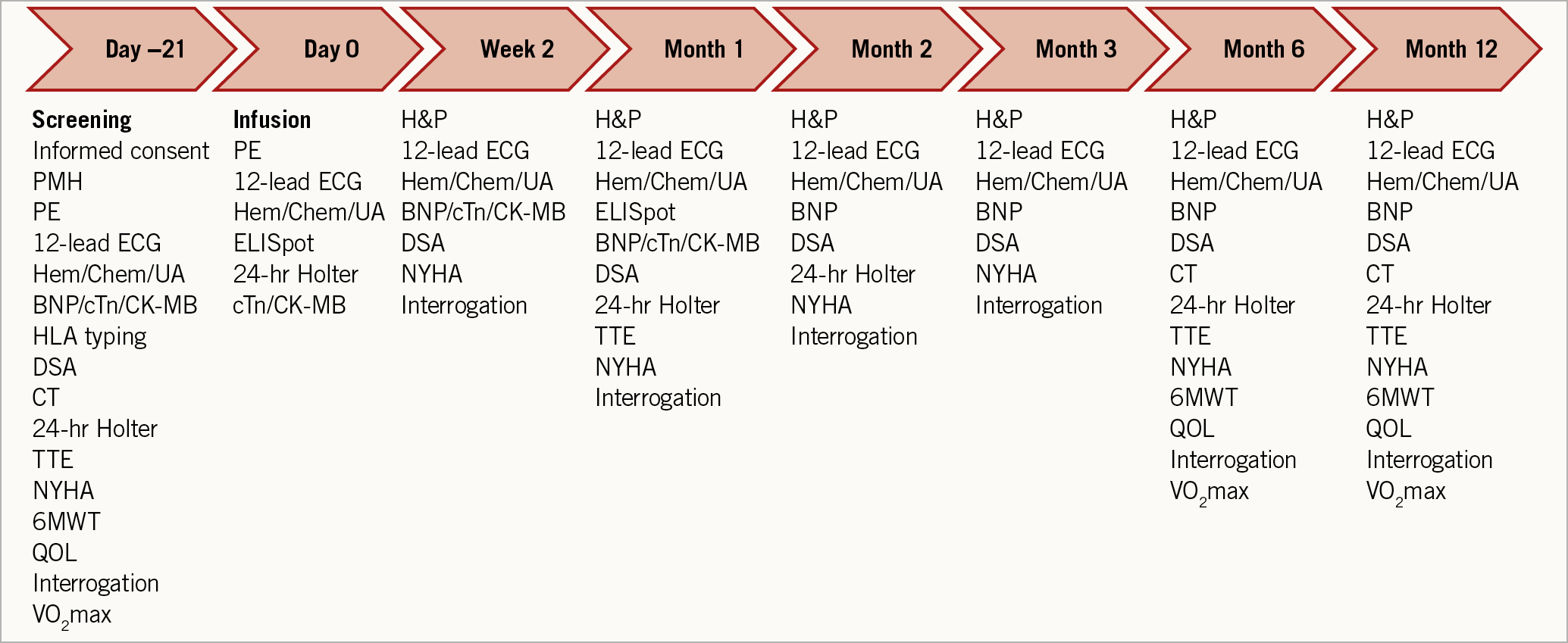
Figure 1. Schedule of assessments.
Within 28 days of the initial baseline assessment, all subjects underwent intracoronary infusion of allogeneic CDCs in an open-label fashion into three coronary territories – left anterior descending, left circumflex and right coronary artery, or the bypass grafts to the corresponding coronary territories. The technical suitability of coronary anatomy for intracoronary infusion was determined by the eligibility committee comprising a team of interventional cardiologists. The enrolled subjects were assigned to four dosing groups in a sequential manner: Group 1 (n=3) allogeneic CDC infusion dose of 12.5 million cells in each cardiac territory; Group 2 (n=3) allogeneic CDC infusion dose of 25 million cells in one cardiac territory and 12.5 million cells in the remaining cardiac territories; Group 3 (n=3) allogeneic CDC infusion dose of 25 million cells in two cardiac territories and 12.5 million cells in the other cardiac territory; and Group 4 (n=5) allogeneic CDC infusion dose of 25 million cells in each cardiac territory (Figure 2). The initial study design included enrolment of an additional 28 patients in a randomised, placebo-controlled manner following the enrolment of the first 14 subjects for the dose-finding study. However, the study sponsor decided not to proceed with the randomised placebo-controlled study following review of the dose-finding study results.
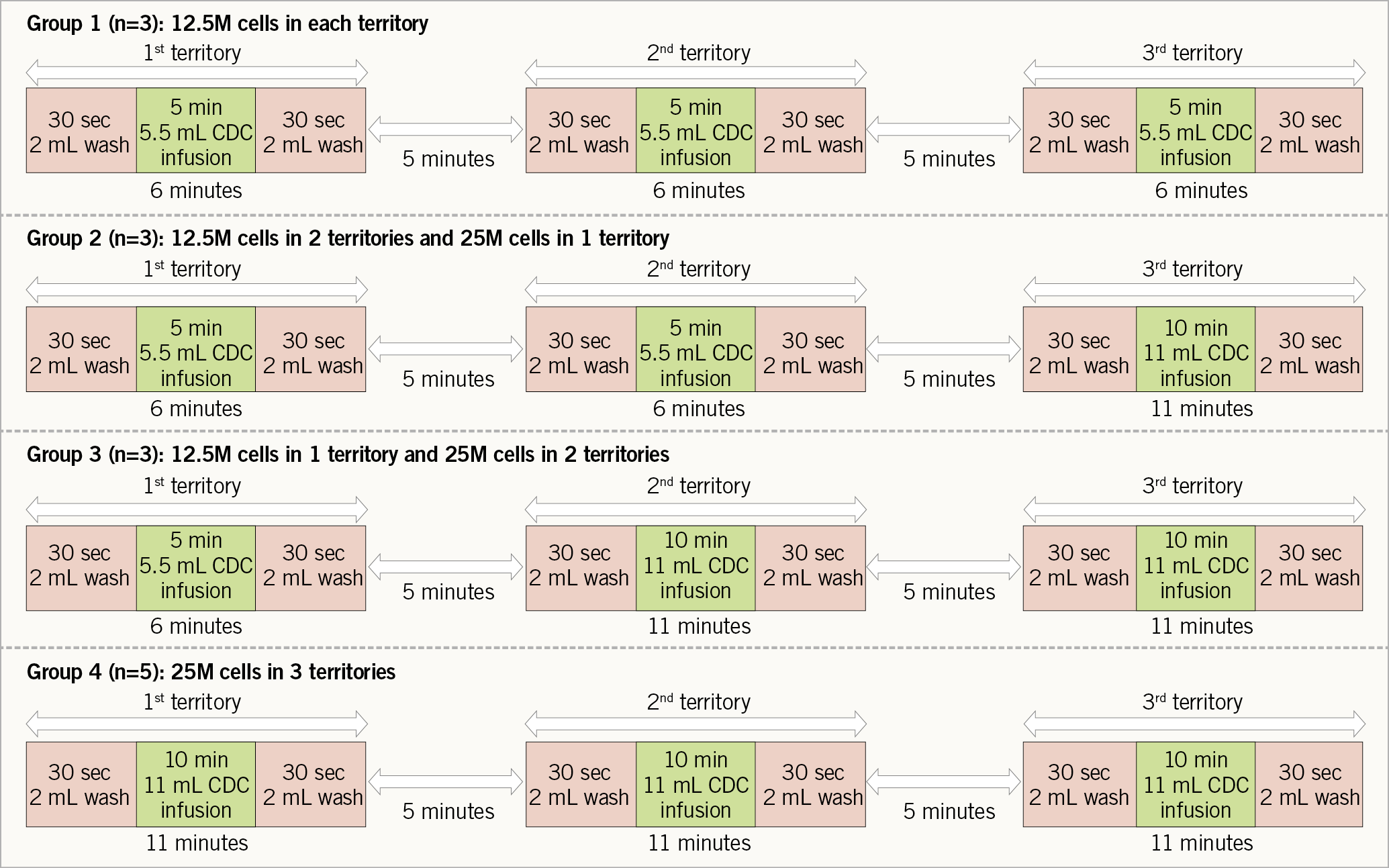
Figure 2. Intracoronary infusion protocol of allogeneic cardiosphere-derived cells.
INFUSION PROTOCOL
Allogeneic CDCs were manufactured as previously described11. Briefly, the tissue derived from donor hearts was cultured as explants. Cardiospheres were generated from the explant-derived cells, and CDCs were expanded from cardiospheres. The detailed infusion protocol is described in Supplementary Appendix 2. Intracoronary infusion of allogeneic CDCs was performed (Figure 2) using Finecross® MG coronary catheters (Terumo Medical Corporation, Somerset, NJ, USA), advanced into the proximal segment of each coronary artery or corresponding bypass graft. After infusion of 2 ml of wash solution over 30 seconds, allogeneic CDCs were infused over five minutes (for 12.5 million cells) or 10 minutes (for 25 million cells), followed by another 2 mL of wash solution over 30 seconds. Once Thrombolysis In Myocardial Infarction (TIMI) 3 flow was confirmed post infusion and following a five-minute interval between infusions in individual coronary territories, the infusion sequence was repeated two more times to treat the remaining coronary territories. Assessment of infusion-induced myocardial injury was performed by serial measurement of ischaemic biomarkers (troponin I [TnI] and creatine kinase-myocardial band [CK-MB]) every eight hours for 16-24 hours post infusion.
FOLLOW-UP
Patients were followed up at 2 weeks and at 1, 2, 3, 6, and 12 months after allogeneic CDC infusion (Figure 1). Adverse events were monitored by the National Heart, Lung, and Blood Institute Gene and Cell Therapy Data and Safety Monitoring Board. Host immunologic response to donor cells was assessed in two ways: 1) for monitoring humoral immunity, single antigen bead testing to detect donor-specific antibodies (Texas Medical Specialty, Inc., Dallas, TX, USA); 2) for monitoring cellular immunity, ELISpot assay (Cellular Technology Ltd., Shaker Heights, OH, USA).
STUDY ENDPOINTS
The primary objective was to determine the safety of allogeneic CDCs administered by multivessel, non-occlusive intracoronary infusion in subjects with HFrEF. The primary safety endpoint was defined as the occurrence of any of the following events during or after intracoronary delivery: (1) new TIMI flow 0-2 or TIMI myocardial perfusion grade 0-2, noted immediately following infusion of allogeneic CDCs and persisting >3 min after cell infusion, despite intracoronary vasodilator administration; (2) acute myocarditis within one month of infusion, possibly attributable to allogeneic CDCs; (3) ventricular tachycardia or fibrillation resulting in death, appropriate discharge of an implantable cardioverter-defibrillator (ICD), or requiring medical intervention, within 72 hours of infusion; (4) sudden unexpected death within 72 hours of infusion; and (5) major adverse cardiac events (death, non-fatal MI and re-hospitalisation for cardiovascular events, including heart failure hospitalisations) within 72 hours of infusion. The diagnosis of acute myocarditis was made based on clinical presentation, with or without a clinically indicated endomyocardial biopsy.
The secondary objective of the study was to explore further the efficacy of allogeneic CDC administration by multivessel, non-occlusive intracoronary infusion in subjects with HFrEF. Several exploratory efficacy endpoints were assessed, including absolute and relative changes in cardiac functional and structural parameters, six-minute walk test (6MWT), maximum oxygen consumption (VO2 max), NYHA class and quality of life (QoL) questionnaires. TTEs were analysed at the Cedars-Sinai Heart Institute by two readers blinded to study visit as well as the cell dosing administered to each subject. The EF was calculated using the biplane Simpson’s method.
STATISTICAL ANALYSIS
The null hypothesis for the primary safety endpoint was that the proportion of subjects experiencing the primary safety endpoint (ps) was >0.20. The null hypothesis was tested against the one-sided alternative ps <0.20 using a 0.05 significance level. Null hypotheses of change from baseline in efficacy parameters = 0 were tested against two-sided alternatives using signed-rank tests, i.e., only subjects with both “pre” (baseline) and “post” (six- or 12-month) data were included. There were no imputations for missing data. The analyses only included subjects who had data at both baseline and the time point being analysed. A two-sided exact binomial test was used to test the null hypothesis that the probability of improvement in NYHA class was = 0.5. All efficacy analyses were carried out using 0.05 significance levels. No adjustments for multiple efficacy endpoints were applied since the efficacy analysis was exploratory and hypothesis-generating. Continuous measures are presented as mean±standard deviation (SD) in the text and Tables and as mean±standard error (SEM) in the Figures. Due to small sample sizes within dose groups, efficacy results are presented as pooled across dose groups.
Results
PATIENTS
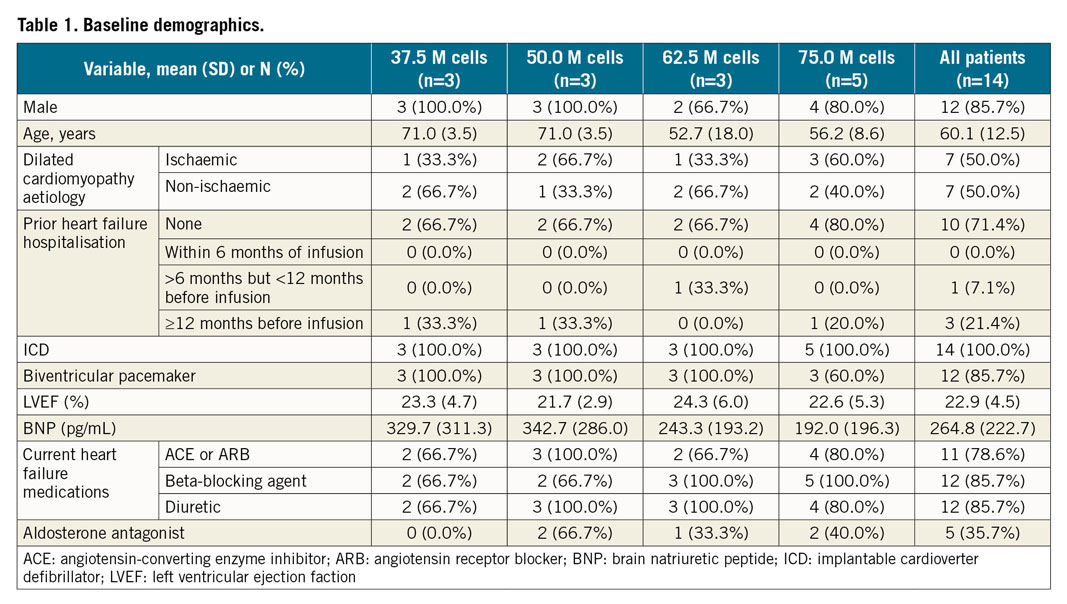
Of 24 patients screened for enrolment, 14 were enrolled in the study (Figure 3). Baseline characteristics of the enrolled patients are summarised in Table 1. The mean EF was 22.9±4.5% and left ventricular (LV) end-diastolic diameter was 69.3±8.9 mm. The aetiology of cardiomyopathy was evenly divided between ischaemic (n=7) and non-ischaemic (n=7). One patient was diagnosed with dilated cardiomyopathy nine months prior to enrolment; the remaining 13 patients had a diagnosis of dilated cardiomyopathy for at least one year prior to enrolment. Six patients underwent an invasive coronary angiogram and four patients underwent a non-invasive CT coronary angiogram within 30 days of the procedure, and four patients underwent an invasive coronary angiogram between one and three months prior to the procedure.
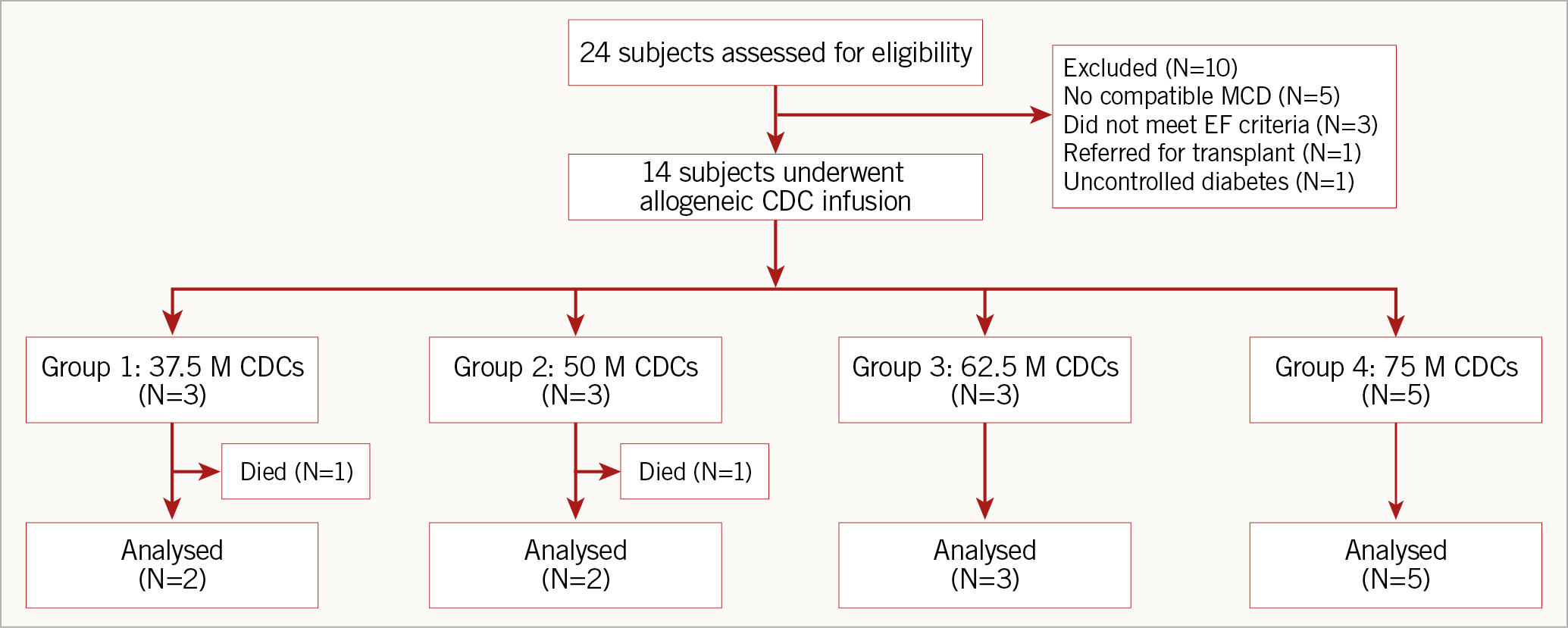
Figure 3. Patient enrolment.
PRODUCT
Donor CDCs were tested against release specifications for allogeneic CDCs and found to meet identity and purity criteria by flow cytometry (>90% CD105+, <10% CD45+), potency criteria by TTE (≥4% EF improvement when administered in immunosuppressed mice), and safety criteria (e.g., viral, sterility, mycoplasma, endotoxin)11.
SAFETY
Multivessel intracoronary infusion of allogeneic CDCs was successfully completed in all 14 subjects. No primary safety events were observed. TnI elevations within 24 hours post infusion were observed in 8 subjects (Group 1, n=2; Group 2, n=1; Group 3, n=2 and Group 4, n=2), accompanied by normal CK-MB; these elevations were transient, without other associated clinical signs or symptoms of ischaemia. On average, TnI increased from 0.03±0.03 ng/ml before cell infusion to a peak of 0.12±0.18 ng/ml at 16 hours post infusion, with full resolution by 14 days (0.03±0.02 ng/ml). Six subjects experienced a total of nine secondary safety events within 12 months of follow-up (Supplementary Table 1). Two subjects died of cardiac failure due to progression of disease nine and 12 months following infusion. No significant tachyarrhythmias or bradyarrhythmias were noted on 24-hour ambulatory ECG monitoring during follow-up.
IMMUNOGENICITY
No development of cellular immunity to infused cells was observed; alloreactive responses to infused cell lines were all negative at one month post infusion by ELISpot testing for 13 of the 14 subjects with available blood samples. Two patients (14%) developed de novo donor-specific antibodies to donor-specific human leucocyte antigen (HLA) (defined by mean fluorescence intensity >5,000 in the single antigen bead assay12) at two and three months post infusion. In both cases the humoral immune response was transient and had resolved by six months post infusion.
EFFICACY
The impact of allogeneic CDC infusion on cardiac function was assessed by TTE at six and 12 months (Table 2, Figure 4). Compared to baseline, there was an improvement at six months in LVEF (26.8±7.2% vs 22.9±4.5%, mean change 3.8±4.9%, p=0.023) and a trend towards improvement in fractional shortening (13.8±6.2% vs 10.3±5.0%, mean change 3.0±4.6%, p=0.061). There was a reversal of LV remodelling at six months, manifested as a decrease in end-systolic volume (LVESV) (177.8±64.2 vs 139.5±46.2 cm3, mean change −38.8±55.7 cm3, p=0.034) and a non-statistically significant decrease in end-diastolic volume (LVEDV) (231.8±103.6 vs 191.5±59.3 cm3, mean change −40.7±72.0 cm3, p=0.077). At 12 months, the improvement in LVEF, compared to baseline, continued to be significant (28.3±8.8% vs 22.9±4.5%, mean change 4.7±6.9%, p=0.029); however, the changes in the other parameters of LV function and remodelling were not significant at 12 months (fractional shortening [p=0.11], LVESV [p=0.26] or LVEDV [p=0.21]) (Figure 4). No patient underwent coronary revascularisation or biventricular ICD placement during the study period. There were no changes in cardioprotective medications made during the study.
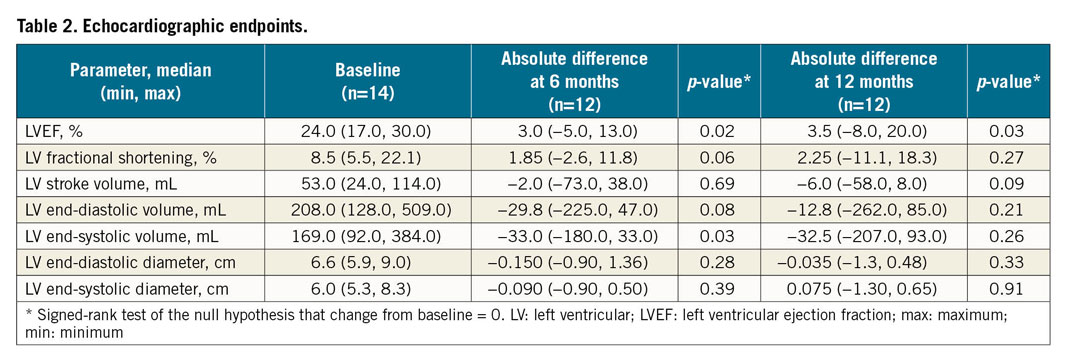
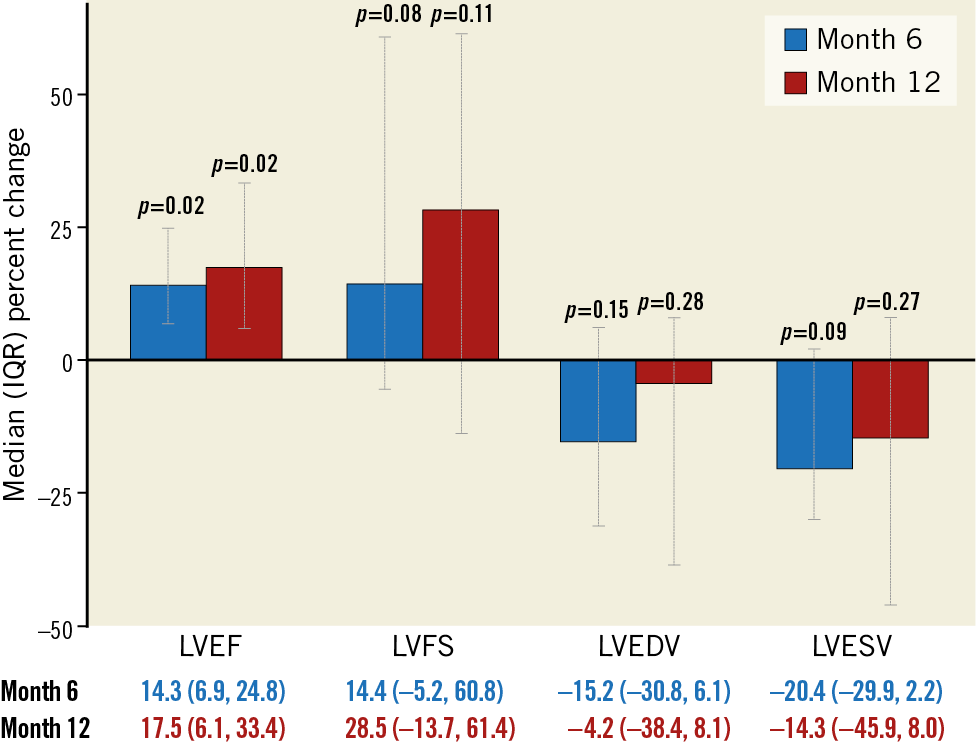
Figure 4. Echocardiographic endpoints at six and 12 months following intracoronary cell infusion.
Supplementary Table 2 summarises the secondary efficacy endpoints. Compared to baseline, there was an improvement in NYHA functional class at six months (p=0.006, compared to baseline) (Figure 5A). By 12 months, the changes in NYHA remained significant in the high-dose group (Figure 5B), but not when patients from all dosage groups were pooled (Figure 5A). While we did not observe a relationship between the CDC dose and LVEF at 12 months, there was a trend towards improvement in 6MWT and NYHA class with increasing CDC doses (Supplementary Figure 1A-Supplementary Figure 1C). Measures of health-related QoL scores improved at six months: Minnesota Living with Heart Failure Questionnaire (MLFHQ) scores decreased (25.1±23.3 vs 50.9±29.9, mean change −21.3±23.6, p=0.012) and SF-36 physical functioning score increased (59.6±21.4 vs 44.6±26.3, mean change 13.3±19.6, p=0.038) over the six months of follow-up. At 12 months, the change in the QoL scores was not significant.
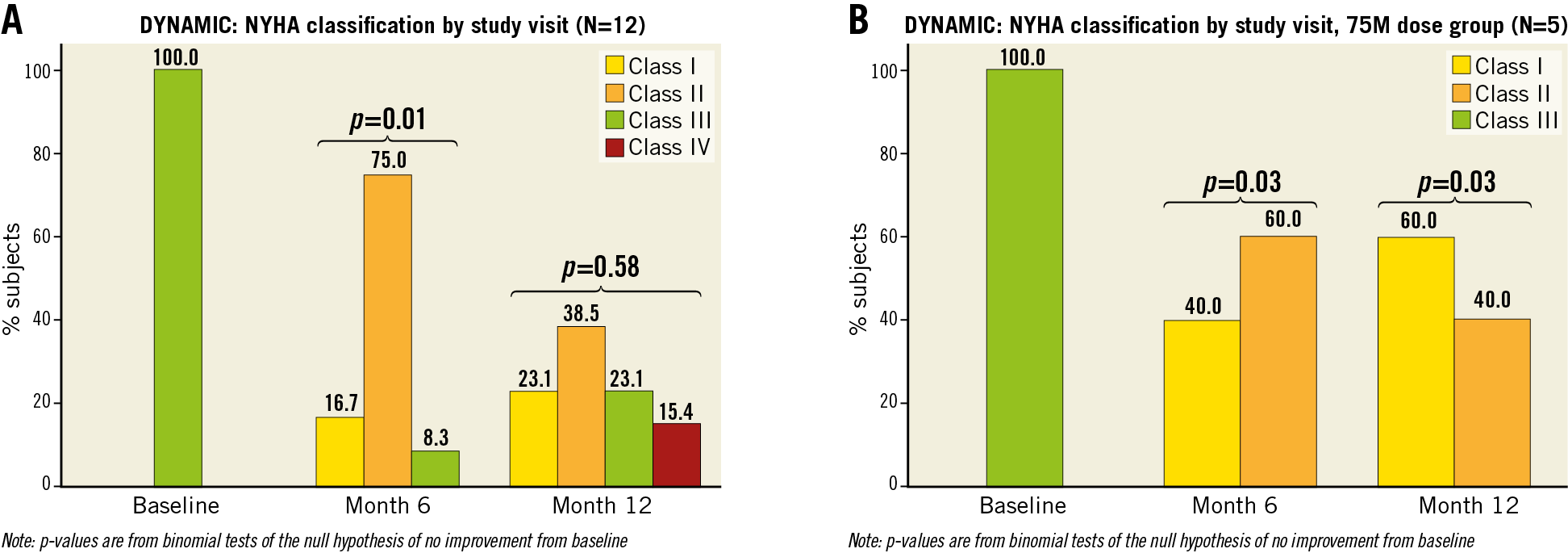
Figure 5. New York Heart Association class at baseline, six and 12 months. A) In the overall cohort. B) In the high-dose (75 million cells) group.
Discussion
The DYNAMIC study design has several unique features. We administered cells derived from unrelated donor (i.e., allogeneic) hearts, building upon our previous work demonstrating safety and efficacy of allogeneic CDCs in animal models of ischaemic cardiomyopathy3,4,5 and in human subjects with MI (Makkar R. ALLogeneic Heart STem Cells to Achieve Myocardial Regeneration [ALLSTAR]: the One Year Phase I Results. Presented at AHA 2014 Scientific Sessions and Resuscitation Science Symposium, Chicago, IL, USA, 25 November 2014). Potential clinical validation of allogeneic cells would obviate the major technical, timing, logistic and economic constraints associated with autologous therapy and facilitate broad clinical adoption of cell therapy. We used a sequential three-vessel non-occlusive intracoronary delivery strategy which has been validated previously in a clinically relevant large animal model13,14,15. This delivery method is technically straightforward (without the need for specialised injection catheters), enables administration of higher cell dosages (compared to single-artery infusion), and achieves broad coverage of the myocardium. We used a “non-occlusive” technique for cell administration in this study, instead of the “stop-flow” technique. A randomised trial of 34 patients receiving intracoronary stem cell infusion by “stop-flow” or “non-occlusive” technique demonstrated that there was no difference in cell retention with either technique; however, the risk of ventricular arrhythmias or intolerance was significantly decreased with the “non-occlusive” technique16.
The principal finding from DYNAMIC is that non-occlusive multivessel intracoronary infusion of allogeneic CDCs in an advanced HFrEF population is feasible and safe. No pre-specified primary safety endpoint events were observed, and no serious adverse events were classified as at least possibly related to allogeneic CDCs or the infusion procedure. While we observed modest, transient increases of TnI (but not CK-MB) in 8/14 infused patients, the incidence of such low-level “leaks” falls well within the range associated with percutaneous coronary interventions17. The troponin leak was noted in all dosing groups undergoing CDC administration; thus, there did not seem to be a correlation between the CDC dose and troponin leak. Regarding host allosensitisation to donor cells, we observed a limited (in 2/14 patients) transient humoral immune response, and a complete absence of cellular immune response. The lack of host memory response to donor cells is particularly relevant in patients with advanced HF, as potential allosensitisation of these patients could complicate future cardiac transplantation (if the CDC donor and organ donor share similar HLA haplotypes)18.
Despite the small sample size and lack of a control group, the trial also demonstrated concordant efficacy signals, especially evident at six months. Treated patients exhibited improvements in global systolic function (LVEF) and reverse remodelling (LVESV). All patients enrolled in the study had been on maximum tolerated medical and device-based therapy for at least three months prior to enrolment. Thus, it is unlikely that the improvement in EF was influenced by the cardioprotective medications or biventricular pacemaker/ICD implantation. There appears to be an improvement in functional status (NYHA class) and quality of life (MLFHQ and SF-36) at six months post infusion; however, the open-label design of the study and lack of a control group could have influenced the improvement in the functional class and quality of life parameters. Except for persistent improvement in LVEF, the other efficacy parameters were no longer significant at 12 months. This is probably related to continued progression of advanced cardiomyopathy. The death of two subjects (14%) during the study period is consistent with advanced HFrEF, with an expected one-year mortality rate of up to 30-35%19. Since the benefits of allogeneic CDC therapy were arguably greater at six months than at 12 months (unlike our previous experience with autologous CDCs in asymptomatic post-MI patients)1,2, repeat dosing of allogeneic CDCs may be an alternative to produce durable sustained, and perhaps even cumulative, benefits of cell therapy5,20.
Limitations
While we did not observe any safety concerns with triple vessel infusion of allogeneic CDCs in patients with advanced cardiomyopathy, the study is limited by small sample size, an open-label design and lack of a control group. Thus, the safety results are preliminary and need to be verified in larger patient populations. In the randomised, placebo-controlled trial of allogeneic CDCs versus placebo for post-MI left ventricular dysfunction (ALLSTAR trial) (Makkar R. ALLogeneic Heart STem Cells to Achieve Myocardial Regeneration [ALLSTAR]: the One Year Phase I Results. Presented at AHA 2014 Scientific Sessions and Resuscitation Science Symposium, Chicago, IL, USA, 25 November 2014), administration of CDCs was not associated with decrease in scar size or improvement in ejection fraction. Thus, the efficacy signals reported in the open-label DYNAMIC study will need to be reproduced in larger, placebo-controlled studies.
Conclusions
In summary, global intracoronary infusion of up to 75 million allogeneic CDCs in patients with HFrEF was feasible, safe and possibly efficacious. Whether allogeneic CDCs offer patients with advanced HF a regenerative and reparative option in addition to the currently available approaches remains to be determined. The favourable safety profile observed in this trial, in conjunction with the hints of efficacy, support proceeding to a randomised, double-blind, placebo-controlled trial of allogeneic CDCs for patients with advanced heart failure.
|
Impact on daily practice In patients with advanced HFrEF, non-occlusive multivessel intracoronary infusion of allogeneic CDCs is feasible and safe. Despite the small sample size and lack of a control group, the trial also demonstrated concordant efficacy signals, especially evident at six months. The favourable safety profile, in conjunction with the hints of efficacy, support proceeding to a randomised, placebo-controlled trial of allogeneic CDCs for patients with advanced HFrEF. |
Acknowledgements
We thank Tracey Earley, Michelle Domingo, Russell Haber and Jonathan Dahan for assistance with patient recruitment, follow-up and regulatory filings; Michelle Kreke, Jeffrey Wells and Kathleen De Guzman for cell manufacturing; and Mark Aminzadeh for having performed the preclinical studies motivating the present study.
Funding
National Institutes of Health (HL095203).
Conflict of interest statement
E. Marban owns founder’s equity in Capricor and serves as unpaid scientific advisor to Capricor. R. Smith, L. Marbán and D. Ascheim are employees of Capricor. K. Malliaras and J.M. Pogoda are paid consultants to Capricor. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

