Abstract
Aims: For appropriately selected patients with severe mitral regurgitation, percutaneous mitral valve repair with the MitraClip® system is a promising alternative to open chest surgery. The procedure requires transoesophageal echocardiographic guidance and is performed under general anaesthesia. However, many patients undergoing percutaneous repair are at high risk for complications related to anaesthesia. We report our initial experience in the use of the MitraClip® system under deep sedation and local anaesthesia in five consecutive cases.
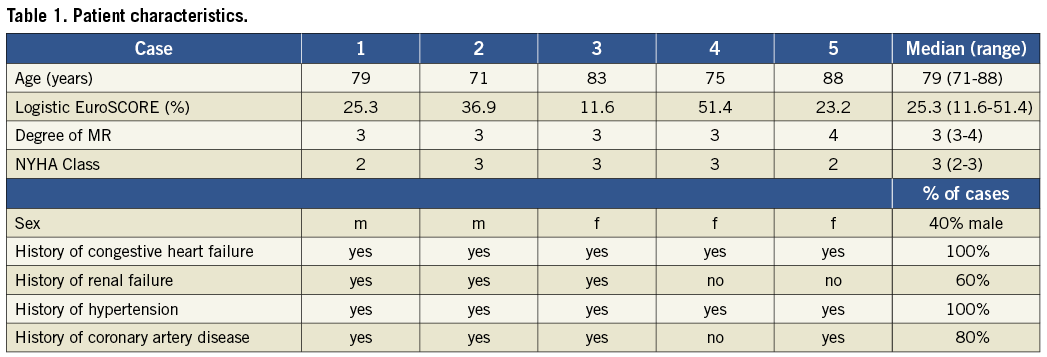
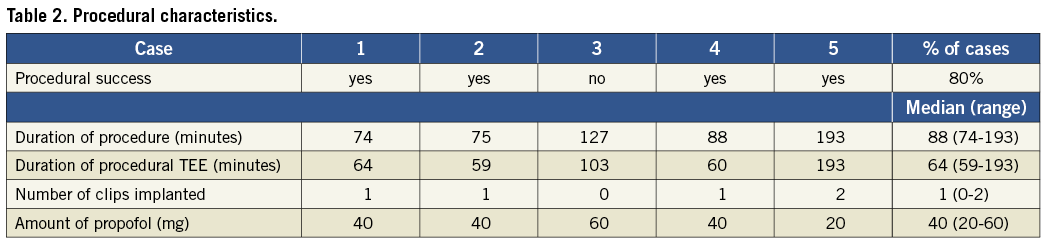
Methods and results: Five patients (two male, three female), median age 79 years (range 71 to 88 years), four with moderate to severe mitral regurgitation suitable for percutaneous repair, underwent the MitraClip® procedure under local anaesthesia and deep sedation. All procedures were completed without general anaesthesia. All patients received 2 mg of midazolam, and propofol was administered according to response during the course of the procedure with 20-60 mg required per case. The median duration of the procedures was 88 (74 to 193) minutes, and the median duration of procedural TEE was 64 (59 to 193) minutes. Four of five procedures were carried out successfully. Three patients required one clip and one patient required two clips. In one patient, the clip was eventually withdrawn and not implanted because it did not lead to an adequate reduction of mitral insufficiency.
Conclusions: The implantation of a MitraClip® is feasible under local anaesthesia and sedation. In patients at high risk for complications related to general anaesthesia, percutaneous mitral valve repair under local anaesthesia may be a viable alternative.
Introduction
For patients with severe mitral regurgitation, the standard therapy for mitral regurgitation (MR) is open surgical repair or replacement. By adapting an established surgical technique (Alfieri edge-to-edge stitch) to a catheter-based approach, the MitraClip® (Abbott Laboratories, Abbott Park, IL, USA) has emerged as a potential percutaneous means of mitral valve repair in appropriately selected patients1,2. The safety and efficacy of the system have been shown in several clinical trials, and it received the CE mark in 20082-4.
The MitraClip® procedure is typically performed using general anaesthesia, as it can be a lengthy procedure requiring transoesophageal echocardiographic (TEE) guidance throughout its duration. However, there is a steep learning curve to percutaneous mitral repair and, with increasing experience, procedure time can often be reduced substantially. Reduced procedure times may afford avoidance of general anaesthesia2,3,5, and this may have particular advantages in patients at high risk for general anaesthesia. We report five consecutive cases of MitraClip® percutaneous mitral valve repair performed under deep sedation and local anaesthesia.
Methods
As part of the screening process, the severity of mitral regurgitation as well as its mechanisms and the mitral valve morphology were assessed using TEE and transthoracic echocardiography. We planned on excluding patients who could not tolerate the 3-D TEE probe easily during the screening process. However, no patient was excluded for that reason. Once it was confirmed that each patient’s mitral valve anatomy would be suitable for percutaneous repair with the MitraClip®, the cases were scheduled to be performed under deep sedation and local anaesthesia. To ensure safety and the possibility to establish deeper sedation and intubation if necessary, anaesthesiology stand-by was available throughout the procedure.
Detailed descriptions of the MitraClip® device and procedural technique have been published previously2,3. Transseptal puncture was performed via femoral venous access and with TEE guidance. Then, the 24 Fr guide catheter of the MitraClip® system was placed in the left atrium and the clip delivery system was inserted and brought to the mitral valve, where the leaflets were then grasped, and the clip was deployed. A second clip could be implanted before the guide catheter and TEE probe were withdrawn and the femoral access site was sutured. To reduce the duration of the procedure and procedural TEE, we prepared the clip delivery system before the guide catheter was successfully placed in the left atrium.
Our anaesthetic regimen consisted of prilocaine subcutaneously at the femoral access site to achieve local anaesthesia. Before placement of the TEE probe, mild sedation was established with 2 mg of midazolam. As needed, based on patient comfort levels, propofol was administered in 20 mg boluses in order to maintain sedation. All patients were awake at the end of the procedure and could be brought directly to the general ward subsequent to the procedure.
Results
Five patients underwent the procedure under deep sedation and local anaesthesia (Table 1). In four of the five cases, the clip implantation was successful with a 2+ or greater reduction in mitral regurgitation. In three cases one clip was implanted, and in one case two clips were implanted. There was no need for evacuation or intubation. Procedural characteristics are shown in Table 2.
In one case, adequate reduction of MR could not be achieved: while the first clip did not reduce the MR, the resulting transmitral gradient was too high (a mean gradient of 6 mmHg measured with TEE), and the implantation of a second clip could not be taken into consideration because of the risk of functional mitral stenosis. Having repositioned the first clip multiple times without improvement, it was withdrawn and the procedure was terminated.
One patient, who had a history of mitral regurgitation after heart transplantation, died 20 days after the procedure and after a hospital stay of 84 days. He had initially been admitted to hospital due to cardiac decompensation and renal failure. After initiation of dialysis and antibiotic therapy, he underwent the MitraClip® procedure, which was carried out successfully and without complication. Mitral insufficiency was reduced from 3+ to 1+. In the following days, he developed septicaemia from a Clostridium difficile colitis and underwent emergency surgery (subtotal colectomy). He died from multiorgan failure.
Procedural TEE was performed without difficulty in all cases. The median duration of the procedures was 88 minutes, and the median duration of procedural TEE was 64 minutes. With regard to deep sedation no complications occurred, and 2 mg of midazolam were administered in all cases. All patients received propofol: one patient required 60 mg, three patients received 40 mg and one patient required only 20 mg.
Discussion
Percutaneous mitral valve repair has been progressing rapidly over recent years, and currently the MitraClip® system is the most studied device. It has been compared to standard of care, open chest surgery, in a prospective randomised clinical trial (EVEREST II). Initial results of the EVEREST II trial showed that the MitraClip® procedure was potentially safer than surgery (owing primarily to the need for blood products) and not significantly less effective4.
Based on EVEREST II, catheter-based mitral valve repair appears to have an important role with regard to procedural safety, especially in high-risk patients. Additionally, general anaesthesia is not without risk5. In the high risk cohort of EVEREST II, two of the 78 patients enrolled had complications related to general anaesthesia: one laryngeal tear with prolonged ventilation and one periprocedural death owing in part to prolonged anaesthesia6.
In our review of the literature, we found one case report of percutaneous mitral valve repair being performed under remifentanil-based conscious sedation7. Our series demonstrates the feasibility and practicality of deep sedation for catheter-based mitral valve repair even in prolonged cases requiring two clips for successful completion. We consider this a valuable finding, as the particular attractiveness of percutaneous mitral repair is with regard to patients at high risk for general anaesthesia, such as elderly patients, those with severe left ventricular dysfunction or those with severe comorbid disease. In such patients, the ability to perform percutaneous repair with sedation only may represent a significant step forward.
In the current study, all five of our patients suffered from congestive heart failure and presented with a median logistic EuroSCORE of 25.3%. Although there were no absolute contraindications to general anaesthesia for these patients, the potential risks of general anaesthesia are always important considerations. We attempted these procedures under deep sedation to minimise any potential risk as well as to establish feasibility of deep sedation for this procedure in patients who could, if necessary, undergo general anaesthesia.
With one unsuccessful case and one two-clip case, we encountered two unexpectedly lengthy procedures (two and three hours respectively). Anaesthesiology stand-by should be standard, and the need for deeper sedation may arise during a prolonged procedure. The safety of midazolam/propofol for longer cases is supported in a recent study by Wutzler et al in which 316 patients underwent atrial fibrillation ablation under sedation with midazolam/propofol. In this study, the procedure time was 235±48 minutes, with midazolam doses of 9.5±3 mg and propofol doses of 1.125±684 mg with no complications noted8.
In our experience, the patients tolerated the TEE probe for up to three hours without difficulty. Although the procedure time for the MitraClip® can vary dramatically, operator experience has consistently been shown to result in improved procedure times2. Shorter cases can be expected to improve further the likelihood of success without general anaesthesia. Furthermore, as these patients could go directly to the general ward after the procedure with no need for monitoring on an intensive or intermediate care unit, patient through-put also stands to benefit from deep sedation.
A series such as this has of course certain limitations. First, it is difficult to generalise from a small, single-centre experience. While this is undeniable, we believe that the procedural learning curve with this procedure is generalisable, and shorter procedure times should allow for deep sedation in any centre. Second, given the fact that both midazolam and propofol have amnestic effects, we cannot state definitively that the patients experienced “total” comfort during the procedure. However, no patient experienced objective signs of discomfort, and the doses of midazolam/propofol were sufficiently low to allow for further dosing if indicated. Lastly, although deep sedation seems an attractive alternative to general anaesthesia where applicable, a larger scale trial is warranted to prove its ultimate value.
Conclusions
Percutaneous mitral valve repair using the MitraClip® system can be performed under local anaesthesia. At least for a subset of patients, the TEE guidance for and implantation of the MitraClip® do not necessarily require general anaesthesia or complete immobilisation. For patients at high risk for anaesthesia-related complications, percutaneous mitral valve repair under deep sedation and local anaesthesia appears to be a viable alternative. With further study, it may prove to be a safer alternative for this subset of patients.
Conflict of interest statement
D. H. Steinberg has served as consultant to Abbott. All other authors have no conflicts of interest to declare.

