Abstract
Aims: Percutaneous MitraClip therapy has been shown to be safe and efficacious in mitral regurgitation (MR). Our aim was to describe early outcomes in patients from the Asia-Pacific region.
Methods and results: The MitraClip Asia-Pacific Registry (MARS) includes data from eight different centres in five countries in the Asia-Pacific region. The primary efficacy outcome was reduction in MR to ≤2+ at 30 days. The safety outcome was 30-day freedom from major adverse events (MAE), defined as the composite of death, myocardial infarction, non-elective cardiac surgery, renal failure, transfusion of ≥2 units of blood, ventilation for >48 hours, septicaemia, and new onset atrial fibrillation. A total of 142 patients underwent the MitraClip procedure from February 2011 to October 2013. Fifty-three point five percent (76) of patients had functional MR, 45.8% (65) had degenerative MR and 0.7% (1) had mixed MR. The acute procedural success rate was 93.7% (133). Thirty-one point seven percent of the patients were in NYHA Class I-II at baseline, compared to 82.1% at 30 days (p<0.001). Zero percent (0) of the patients had ≤2+ MR at baseline compared to 76.8% (109) at 30 days (p<0.001).
Conclusions: Results from the Asia-Pacific region show that the MitraClip procedure is effective in reducing mitral regurgitation and has favourable short-term safety outcomes.
Introduction
The MitraClip® (Abbott Vascular, Santa Clara, CA, USA) is the first commercially available therapy for the percutaneous repair of mitral regurgitation (MR). It mimics, but is not identical to, the surgical Alfieri stitch concept for the repair of the mitral valve1,2. The landmark EVEREST II trial showed that the therapy, when used in patients considered suitable for conventional surgery, was safer, but less efficacious3. The device was first Conformité Européenne (CE) marked and approved for use in Europe in 2008. Since then, the commercial use of the device has largely been in Europe4-7. In early 2011, the device was approved for commercial use in Australia, Malaysia and Singapore. Initial adoption in the Asia-Pacific region was limited by the cost of developing sustainable programmes.
While the Endovascular Valve Edge-to-Edge REpair STudy (EVEREST) II was performed largely in degenerative MR patients, the clinical experience from commercial sites, especially in Europe, has been in patients with functional MR3. Available data from Europe suggest that the device is efficacious, improves symptoms, New York Heart Association (NYHA) functional class, six-minute walk test and quality of life in patients with severe MR4-10. Most of the patients treated in Europe are those with functional MR, the opposite of the distribution seen in EVEREST II.
Limited data on the Asia-Pacific experience have been published, with only case reports in the literature11. With the Asian aversion to open heart surgery and different socio-economic models of healthcare delivery, much remains unclear on the role of the MitraClip in the Asia-Pacific region. In this present study, our aim was to describe the MitraClip experience in the Asia-Pacific region, including 30-day outcomes.
Methods
PATIENTS AND REGISTRY DESIGN
The MitraClip in the Asia-Pacific Registry (MARS) is a multicentre retrospective registry that includes patients treated in Australia, China, Indonesia, Malaysia and Singapore. It currently comprises eight sites in these countries. The MARS registry includes patients treated from February 2011 to October 2013. Patients were offered the therapy as a therapeutic option after assessment by a multidisciplinary team comprising interventional cardiologists, cardiac surgeons, heart failure specialists and echocardiologists. Patients were offered the therapy if they had ≥3+ degenerative or functional MR. For degenerative MR, patients had to have appropriate indications for MV repair according to the American College of Cardiology/American Heart Association guidelines for surgical treatment of MR12. Patients were offered MitraClip therapy if the Heart Team felt that the patient was at high surgical risk. For functional MR, patients were offered the therapy as long as they were symptomatic.
There were no specific anatomic requirements beyond the technical feasibility of grasping the mitral leaflets. In other words, the patients selected were not restricted to those who had central mitral regurgitation involving A2/P2 segments. General contraindications, such as the presence of significant mitral stenosis (mitral valve area <4.0 cm2) and infective endocarditis, etc., were adhered to. Data collected included demographic and clinical characteristics, echocardiographic data (transthoracic [TTE] and transoesophageal echo [TEE]), procedural data and clinical outcomes.
MITRACLIP PROCEDURE
The MitraClip valve repair system is a commercially available percutaneous device used for the repair of significant functional or degenerative mitral regurgitation. Percutaneous MitraClip therapy is usually performed under general anaesthesia, with transoesophageal and fluoroscopic guidance, via a 24 Fr guide catheter from the femoral vein. Details of the procedure have been described elsewhere in detail3,9. Briefly, femoral venous access is obtained, usually via the right common femoral vein. Under precise TEE guidance, transseptal puncture across the interatrial septum is obtained and a stiff guidewire placed in the left upper pulmonary vein. The transseptal sheath is then exchanged for the MitraClip guide which is positioned in the left atrium. The MitraClip Clip Delivery System is delivered to the left atrium. Under careful TEE guidance, the MitraClip is then negotiated to the mitral valve where it is adjusted to the precise position where mitral regurgitation is most severe, or where there is a prolapse or flail section. The clip arms are opened, rotated so that the MitraClip is perpendicular to the line of coaptation and advanced into the left ventricle. The anterior and posterior mitral valve leaflets are then grasped between each clip arm and its associated gripper. The clip arms are apposed, resulting in a dual orifice mitral valve. After verification of reduction of MR, confirmation of leaflet insertion and final arm angle checks, the MitraClip is released from the delivery system and the system removed into the guide. A second clip may be placed to reduce MR further, as appropriate. Once sufficient MR reduction is achieved, the guide is removed from the body.
ECHOCARDIOGRAPHIC PARAMETERS
At baseline, the severity of MR was graded according to guidelines proposed by the American Society of Echocardiography13. MR severity was defined by an integrative approach. Severe MR was defined as a combination of various echocardiographic parameters, including: central and large colour flow jet (area >40% of left atrium [LA]), or with a wall-impinging jet of any size, swirling in the LA; large flow convergence; systolic flow reversal in pulmonary vein flow; vena contracta width >0.7 mm; effective regurgitant orifice area ≥0.4 cm2; regurgitant fraction ≥50% and regurgitant volume ≥60 ml. Moderate-severe MR included characteristics such as vena contracta ≥0.3 cm; effective regurgitant orifice area 0.30-0.39 cm2; regurgitant fraction 40-49% and regurgitant volume 45-59 ml2. The severity of MR post procedure was assessed according to published recommendations14.
The criteria were similar to those of native mitral regurgitation except that vena contracta and effective regurgitant orifice area were not used, as they have not been validated in a double orifice mitral valve. Other echocardiographic parameters measured included left ventricular ejection fraction (LVEF), left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), mitral annulus diameter, LA volumes and pulmonary artery systolic pressure (PASP). The LVEDD and LVESD were measured using M-mode in the parasternal long axis in diastole and systole, respectively. The mitral annulus diameter was measured in the apical four-chamber view at the end of systole. LVEF and LA volumes were measured using the biplane Simpson’s method and LA volumes were indexed to body surface area. PASP was estimated from the maximal velocity of the tricuspid regurgitation jet and the estimated right atrial pressure. These were self-reported by each site before and after the intervention.
Study outcomes
Acute procedural success was defined as successful implantation of one or more clips with immediate reduction of the MR to ≤2+. The primary efficacy outcome was defined as a reduction in grade of MR to ≤2+ at 30 days. Other efficacy outcomes included improvement in New York Heart Association functional class status and improvements in the echocardiographic parameters described above at 30 days. Safety outcomes included in-hospital and 30-day mortality and major adverse events (MAE) including stroke, myocardial infarction, bleeding requiring transfusions >1 unit of blood, septicaemia, reoperation for failed mitral valve procedure, non-elective cardiac surgery for adverse events, renal failure, gastrointestinal complications requiring surgery, ventilation for >48 hours, and new onset of atrial fibrillation.
Statistics
The clinical characteristic profile of the study population was characterised using descriptive statistics. All values were presented as mean and standard deviation for continuous data and frequency and percentage for categorical data. The χ2 test was used to compare categorical variables. For continuous variables, between-group comparison was studied using the independent t-test. Echocardiographic data pre and post clip deployment were compared using the paired t-test. A two-tailed p-value of <0.05 was taken to be statistically significant. All analyses were performed using SPSS version 18 (SPSS Inc., Chicago, IL, USA).
Results
PATIENT CHARACTERISTICS
A total of 145 patients underwent the MitraClip procedure. Of these, 142 patients (97.9%) (mean age 71.4±11.9 years, 91 [64%] males) had one or more clips deployed. These patients underwent the MitraClip procedure during the period from February 2011 to October 2013 and form the basis of the findings. Three other patients underwent the procedure but did not receive a clip, one case due to Chiari network in the right atrium, one case due to a fractured actuator knob and one case due to inability to grasp leaflets. These three patients were excluded from the analysis. The patients were recruited from eight different centres in five countries: Singapore (2 centres, 59 patients), Australia (3 centres, 49 patients), Malaysia (1 centre, 23 patients), Indonesia (1 centre, 7 patients) and China (1 centre, 4 patients). One hundred and fifteen (81%) patients had grade 4+ MR at baseline; the rest had 3+. Seventy-six (53.5%) patients had functional MR, 65 (45.8%) had degenerative and 1 (0.7%) had mixed MR. Ninety-seven (68.3%) of the patients were in NYHA functional Class III/IV, while 45 (31.7%) were in Class I/II. The logistic EuroSCORE and STS score were 16.8±14.6% and 7.4±8.1%, respectively. The LVEF, LVEDD, LVESD, mitral annulus diameter, LA indexed volume and PASP were 48±16%, 5.9±1.0 cm, 4.4±1.2 cm, 3.4±0.6 cm, 77.2±50.5 ml/m2 and 45±21 mmHg, respectively. The rest of the baseline characteristics are shown in Table 1.
PROCEDURAL RESULTS
The average duration of the procedure was 130±98 min. The acute procedural success rate was 93.7% (133/142); six (4.2%) patients had single leaflet detachment, one had a torn chord and there was difficulty in grasping the leaflets in two patients. There was no significant difference in acute procedural success between functional MR (96.1%, 73/76) and degenerative MR (90.8%, 59/65) (p=0.302). Of those without procedural success, two patients passed away in-hospital. Sixty-seven patients (47.2%) had two clips implanted, five (3.5%) had three clips implanted, while the remainder had one clip implanted (49.3%). The mean length of hospital stay was 6.0±7.8 days.
SAFETY OUTCOMES
The 30-day mortality rate was 5.6% (n=8), of which 4.2% (n=6) was in-patient mortality. The 30-day MAE rate was 12.7% (18/142). One patient (0.7%) underwent a mitral valve operation, five patients (3.5%) had blood transfusions of ≥2 units, two patients (1.4%) had sepsis, and one patient (0.7%) had prolonged intubation. In addition, one patient (0.7%) was readmitted for heart failure. There were no cases of MitraClip device embolisation. There was no significant difference in 30-day MAE rate between functional MR (9.2%, 7/76) and degenerative MR (15.4%, 10/65) (p=0.306).
EFFICACY OUTCOMES
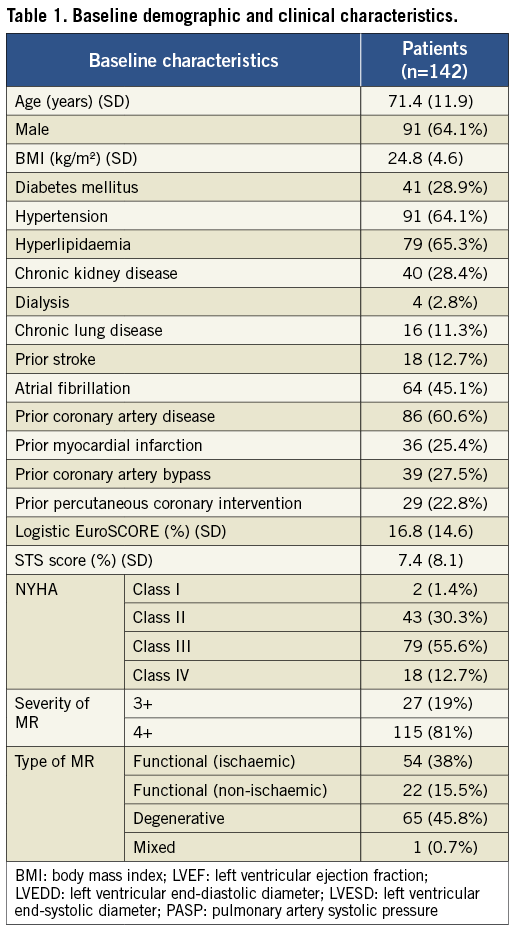
The overall primary efficacy outcome of MR grade ≤2+ at 30 days was 76.8% (n=109). There was significant improvement in MR grade amongst the treated patients at 30 days: 0% of the patients had ≤2+ MR at baseline compared to 76.8% of patients at 30 days (p<0.001) (Figure 1). There was no significant difference in the primary efficacy outcome of MR grade ≤2+ at 30 days between functional MR (81.6%, 62/76) and degenerative MR (72.3%, 47/65) (p=0.228). There was significant improvement in NYHA functional class amongst the successfully treated patients. Eighty-two point one percent of the patients were in Class I-II at 30 days compared to 31.7% at baseline (p<0.001) (Figure 2).
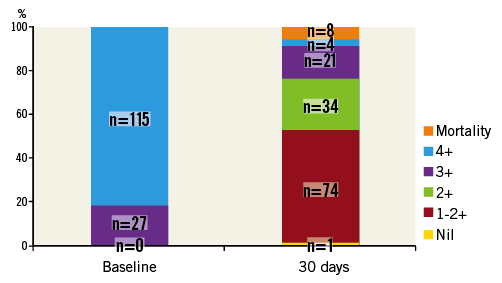
Figure 1. Comparison of mitral regurgitation grade at baseline and at 30 days post MitraClip (numbers in columns indicate number of patients).
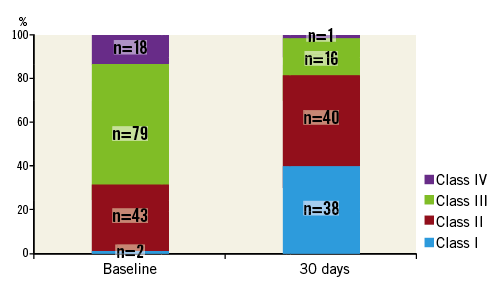
Figure 2. Comparison of New York Heart Association functional class status at baseline and at 30 days post MitraClip (numbers in columns indicate number of patients).
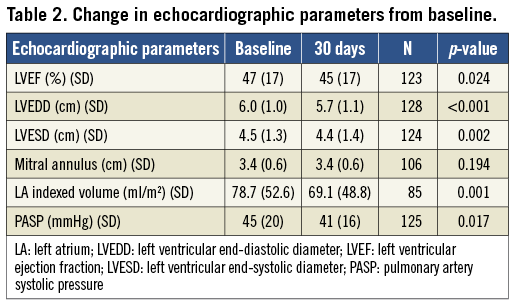
At 30 days, there was a significant reduction in LVEF, LVEDD, LVESD, LA indexed volume and calculated pulmonary artery systolic pressure compared to baseline (Table 2). There was no change in mitral annular diameter. The average mitral valve mean pressure gradient at 30 days was 3.7±1.8 mmHg. Compared to those with >2+ MR at 30 days, those with ≤2+ MR had a significantly smaller baseline LA indexed volume (70±47 vs. 105±61 ml/m2, p=0.012). There were no other significant differences in the rest of the parameters.
Discussion
This is the first study describing the use of MitraClip therapy in the Asia-Pacific region. Despite the early experience across different countries in the region, the MitraClip procedure was safe and efficacious with 76.8% of patients with ≤2+ MR at 30 days. These short-term results have been reassuring despite the steep learning curve and widely different models of healthcare. Acute procedural success (93.7%) is similar to that reported in the German TRAMI study8 (94%) and in the Swiss MitraSwiss study10 (85%), although the larger and more established ACCESS-EU study6 reported rates of 99.6%. Similarly, the MAE rates reported in MARS (12.7%) are comparable to the rates reported in EVEREST II (15%)3. Thirty-day mortality in MARS (5.6%) is also similar to the rates reported in EVEREST II (6%)3, the ACCESS-EU study (3.4%)6 and the MitraSwiss study (4%)10. The mean length of hospital stay (six days) was similar to that reported in ACCESS-EU and MitraSwiss (7.7 and 6.5 days, respectively). There was significant variation in the number of patients treated at each centre, from four to 37. Despite these differences and the limited volume in each individual site, these early outcomes are reassuring and reflect the overall global experience with patient selection and with the procedure itself.
While the device was recently approved by the Food and Drug Administration in the United States (October 2013), commercial experience and literature on the use of the therapy have largely been from Europe. There are important differences between the findings from the European experience and MARS. The ACCESS-EU study is a European prospective, multicentre, non-randomised post-approval study of MitraClip therapy6. Although the device was CE marked from March 2008, the ACCESS-EU registry enrolled patients from April 2009 to April 2011. A total of 567 patients were enrolled at 14 European sites. In comparison, the MARS registry included patients from February 2011 to October 2013 over eight sites, and reported only 142 patients. The characteristics of both groups of patients (MARS vs. ACCESS-EU) are fairly similar for some variables such as age (71.4 vs. 73.7 years) and diabetes mellitus (28.9 vs. 29.6%). However, the mean logistic EuroSCORE in the MARS registry appears to be numerically lower than that in the ACCESS-EU study (16.8 vs. 23.0%). Perhaps most importantly, the Asia-Pacific experience has comparatively more degenerative MR patients than in Europe (45.8 vs. 20.6% in ACCESS-EU8, 33% in TRAMI8, and 38% in MitraSwiss10). The reasons underlying these differences are unclear and merit closer examination. This may relate to the healthcare financing model and cultural characteristics in the region. In Asian countries, for example, the cost of the device may be a significant deterrent as patients may be required to contribute to the cost of the device. This is especially relevant in functional MR where the use of the MitraClip device has not been shown to improve mortality when compared to medical therapy alone. In Australia the device must be funded by individual hospitals as no funding reimbursement system has been established by either the Commonwealth Government or private insurers. Anecdotally, there is also a perception that Asian patients are less inclined to undergo surgery, even when indicated, such as in symptomatic severe MR. These two factors, if true, can certainly contribute to the proportion of patients with degenerative MR.
Like all published series on the MitraClip, the therapy is associated with improvement of MR severity, echocardiographic parameters and functional status5,6,8,10. The MR reduction at 30 days seen in MARS is lower than that reported in the ACCESS-EU study and in the MitraSwiss registry10, with the proportion of patients with MR ≤2+ at 76.8% (vs. 91.2% in ACCESS-EU6 and 87% in MitraSwiss). This probably represents the learning curve and early experience in the region, and is consistent with the longer mean procedure time compared to that reported in ACCESS-EU (130 vs. 100 min)6.
Nevertheless, the therapy is associated with an improvement in NYHA functional class, as shown in Figure 2. This is consistent with improvements in echocardiographic parameters, such as LVEDD, LVESD, LA indexed volume and PASP, seen also in other studies5. These improvements in echocardiographic parameters are probably the result of reduction in volume overload (from the reduction in MR) and the positive initial remodelling of the chambers of the heart. The reduction in LVEF post clip is an expected finding in which the reduction in MR results in improved forward flow. However, the higher afterload conditions of pumping against the aorta result in the appearance of a lower LVEF. Compared to those with >2+ MR at 30 days, those with ≤2+ MR had a significantly smaller baseline LA indexed volume while there were no other significant differences in the rest of the parameters (Table 2). These differences may not manifest sufficiently on account of the small sample size and the short duration of follow-up.
Limitations
The MARS registry has important limitations which must be appreciated in interpreting its findings. First, it is an observational, retrospective study with site self-reported findings. Second, it does not currently have core lab adjudication of echocardiographic findings. Third, it lacks a comparator group of either surgical or medical therapy. Finally, this report only included findings up to 30 days. Longer duration follow-up will be important in determining the impact of the MitraClip therapy.
Conclusions
The MARS registry adds to the growing body of evidence that MitraClip therapy is a safe and efficacious therapeutic option for patients with either functional or degenerative MR. In the Asia-Pacific region, the significant proportion of degenerative MR, in comparison to the commercial experience in Europe, deserves further examination.
Conflict of interest statement
K.K. Yeo, E. Yamen, N. Muda, E. Tay and D. Muller are on the speakers’ bureau for Abbott Vascular. The other authors have no conflicts of interest to declare.

