Abstract
Aims: To describe patient radiation utilisation during transcatheter aortic valve replacement (TAVR) on a series of consecutive patients.
Methods and results: Data on radiation exposure were prospectively collected for consecutive patients undergoing TAVR and percutaneous coronary interventions at our centre. Radiation dose during the procedure was recorded using the US Food and Drug Administration (FDA) reference point (Ka,r) and the dose area product (PKA). In addition to quantifying overall radiation doses during TAVR, radiation exposure during transfemoral (TF) (n=79) and transapical (TA) (n=26) cases was compared. The median radiation dose during TAVR was 1,639 mGy (983-2,420), or 188 (106-321) Gy*cm2. Radiation dose was significantly lower among TA patients using either the reference point (TA: 946 [777-1,261] vs. TF: 1,932 [1,383-2,614] mGy; p<0.001) or the dose area product (TA: 89 [60-115] vs. TF: 236 [164-338] Gy*cm2; p<0.001). Fluoroscopy time was lower for TA patients (TA: 10 [8-11] vs. TF: 30 [24-34] minutes; p<0.001). Operators experience did not affect radiation exposure for TF cases.
Conclusions: Radiation exposure during TAVR appears similar to other percutaneous coronary interventions of moderate complexity. Radiation doses were significantly lower for TA procedures. A higher dose of radiation in TF patients may be related to additional imaging requirements to optimise percutaneous vascular access and closure.
Abbreviations
AVA: aortic valve area
BMI: body mass index
BSA: body surface area
CABG: coronary artery bypass grafting
COPD: chronic obstructive pulmonary disease
E: effective dose
ICRP: International Commission on Radiological Protection
Ka,r: total air kerma measured at the interventional reference point during a procedure
NCRP: National Council on Radiation Protection and Measurements
PARTNER: Placement of Aortic Transcatheter Valves trial
PCI: percutaneous coronary intervention
PKA: total air kerma area product measured during a procedure
QA: quality assessment
TA: transapical
TAVR: transcatheter aortic valve replacement
TEE: transoesophageal echocardiography
TF: transfemoral
Introduction
Recently, transcatheter aortic valve replacement (TAVR) has emerged as a new alternative treatment for patients with severe aortic stenosis who are at “high risk” or deemed inadequate candidates for conventional surgical aortic valve replacement. The feasibility and efficacy of this new technique has been demonstrated in multiple registries and randomised trials.1-11 TAVR typically uses cine-fluoroscopic guidance and adjunctive transoesophageal echocardiography (TEE) for valve assessments and angiographic imaging for access site management. The radiation utilised with cine-fluoroscopic imaging has known potential adverse side effects for both patients and operators.12-14 Dosimetric data are needed to estimate the probability of these events. Currently, the range of radiation doses utilised during TAVR procedures is not well known. The aim of this study was to describe radiation utilisation during TAVR on a series of consecutive patients treated at our institution.
Methods
Consecutive patients undergoing TAVR in our centre were identified, and data elements were collected both prospectively and retrospectively. All patients were treated as part of the randomised PARTNER trial with the Edwards SAPIEN™ valve (Edwards Lifesciences, Irvine, CA, USA). Two hybrid cathlab suites (Siemens, Erlangen, Germany) were used. Clinical follow-up was available at one and six months after the procedure. As part of the laboratory’s quality assessment (QA) process, data on radiation exposure from all procedures is routinely collected and entered in a database. All interventional fluoroscopes are equipped with integrated dosimetry instrumentation. The performance of this instrumentation is verified semi-annually as part of the laboratory’s QA programme. Baseline characteristics, procedural events and radiation doses were collected in 105 patients who underwent TAVR. Radiation dose during the procedure was recorded using the FDA reference point (Ka,r) and the air kinetic energy released per unit mass (kerma) area product (PKA), also known as the dose area product (DAP) as previously described.12-14 Total fluoroscopic time was also ascertained.
Patient radiation exposure during percutaneous coronary interventions (PCI) done in a different cathlab suite (GE Healthcare, Chalfont St-Giles, UK) between January 2010 and January 2011 was also analysed and reported for comparison. A different suite was used because of the low number of PCI performed in the two hybrid suites used mostly for TAVR and peripheral interventions.
Results are presented as medians with interquartile ranges. Radiation doses during TAVR were compared between transfemoral (TF) and transapical (TA) approaches. An additional comparison was made between TF cases performed early on in our experience versus cases performed later in our TF experience. Comparisons between groups were done using the Mann-Whitney U test. Pearson’s correlation was used to identify univariate predictors of radiation use. SPSS 18.0 software (SPSS Inc., Chicago, IL, USA) was used for all statistical analysis.
Results
Baseline characteristics from the 105 identified patients are presented in Table 1. Seventy-nine patients underwent the procedure via a TF approach and 26 via a TA approach. Thirty-seven patients (35%) received a 23 mm valve and 68 patients (65%) received a 26 mm valve. All patients had intra-operative TEE. The median radiation dose during TAVR for all procedures was Ka,r=1,639 (983-2,420) mGy, and P KA=188 (106-321) Gy*cm2.
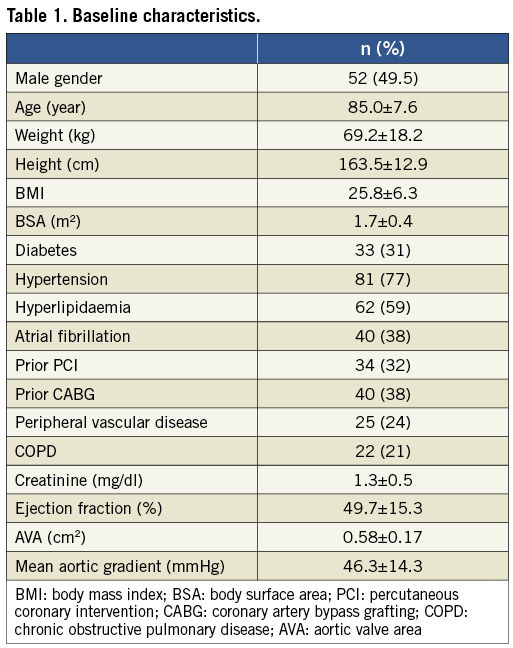
Radiation doses and fluoroscopic time were significantly lower for TA patients compared to TF patients (Table 2). Three patients (all TF) had Ka,r above 5,000 mGy; two had procedure-related complications (one transcatheter heart valve embolisation and one vascular perforation treated with a covered stent), and the other patient was morbidly obese. No clinical complication related to radiation was seen in those patients at their one- and six-month follow-up visit.
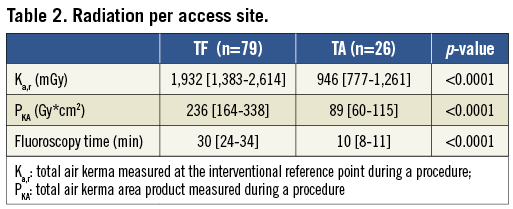
Patient weight, body surface area (BSA) and body mass index (BMI) correlated with increased radiation use in TF patients. Among TA cases, increased weight, BSA, BMI and height were identified as correlates of greater radiation doses. As expected, longer fluoroscopic time was also an indicator of greater radiation dose for both approaches (Table 3).
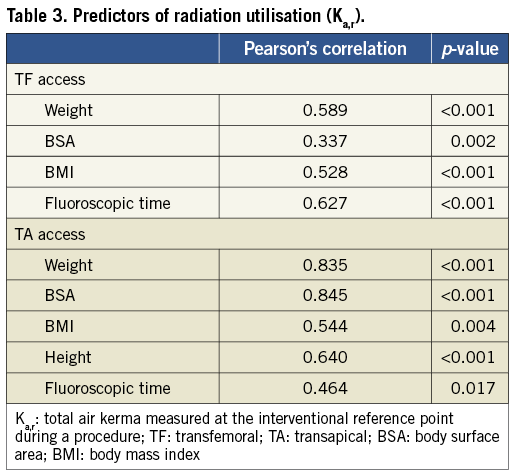
To assess the impact of a learning curve and increasing experience with the procedure, TF cases were separated into two groups –the early (40 first cases) and late (last 39 cases). There were no significant differences in Ka,r, PKA and fluoroscopic time between the two groups (Table 4).
A total of 637 consecutive PCI were analysed for patient radiation exposure. This patient population was markedly different than the TAVR population (Table 5). The median radiation dose for PCI was Ka,r=2,723 (1,631-4,347) mGy and PKA=162 (101-257) Gy*cm2. For PCI, median fluoroscopy time was shorter and median P KA was less than for TAVR while median Ka,r was greater (Table 5).
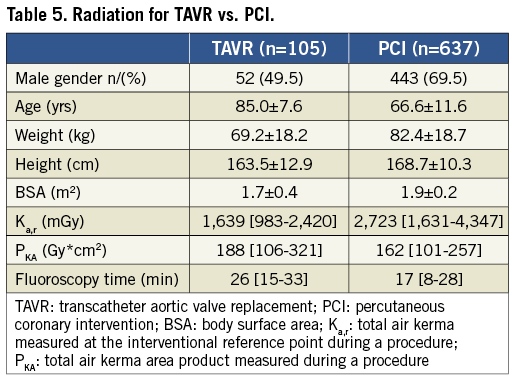
Discussion
This study represents the largest cohort of patients systematically evaluated for radiation exposure during TAVR. The principal findings of the present analysis are: 1) Radiation use during TAVR is within the acceptable range of radiation compared to other percutaneous coronary procedures; 2) The TA approach compared to TF approach was associated with a lower dose of radiation to the patient; 3) Higher body weight and BMI were associated with greater amounts of radiation.
TAVR is an emerging procedure for the treatment of aortic stenosis that is at present an alternative to open heart surgery for higher risk patients. Radiation exposure during TAVR is something that is ill defined and is relevant particularly if this technique expands to lower risk and younger patients. Known side effects of radiation include hair loss, skin damage (related to Ka,r) and risk for subsequent development of neoplasia (related to PKA).12-14 Signorotto et al reported radiation exposure of cardiac procedures including 76 TAVR patients, but only seven TA cases.15 They observed a mean PKA (dose area product) of 171 Gy*cm2 for complex PCI and 259 Gy*cm2 for TAVR. The PKA observed in our study are lower than those reported by Signorotto et al, possibly because of a larger proportion of TA cases.
We were able to demonstrate that the radiation use and fluoroscopic time is significantly lower for TA cases. Consistent with the present report, Ewe et al also demonstrated that fluoroscopic time was significantly longer for TF (12 vs. 5 minutes, p<0.001).16 Our observation is likely due to the access and closure of the femoral artery, which requires extra fluoroscopy time, compared to the surgical access of the ventricle apex. However, Ewe et al used surgical access and repair of the femoral artery for TF cases and explained the shorter fluoroscopic time for TA by an easier implantation. The mean fluoroscopic time for TF cases in our study is markedly longer than what was reported by Ewe et al. Several factors could explain this finding. The systematic use of an adjunctive technique at the time of percutaneous vascular closure (crossover balloon occlusion technique)17 during TF cases could have played an important role. TA cases in our study also had longer fluoroscopic time than reported by Ewe et al. Since this represents the early experience of both centres, there is no evident factor to explain such a difference. Furthermore, the occurrence of two major vascular complications in the TF group significantly prolonged procedural and fluoroscopic time, and therefore, radiation. Indeed, the management of major complications during TF cases often requires the use of fluoroscopy, whereas the management of complications during TA, such as bleeding of the apex, tamponade or right ventricle perforation requires urgent surgical treatment that does not involve fluoroscopic guidance. Finally, the deployment of the valve was performed under cine rather than fluoroscopy for all patients in the current report. These runs are often 15 seconds and more, and deliver significant radiation.
As expected, weight and BMI were found to be predictors of radiation utilisation but not of fluoroscopic time. This can be explained by the fact that larger patients require an increased amount of radiation to generate adequate imaging, but this does not mean a longer procedure. A recent study evaluated determinants of radiation dose in PCI procedures in a single US centre.18 The median radiation dose observed on 1,827 procedures was 1,480 mGy. As in our study, they found that increased BMI and male gender was associated with a greater radiation dose. Other predictive factors that were identified are more complex PCI, prior coronary artery bypass grafting (CABG), primary PCI, peripheral vascular disease and use of the lateral projection. These factors are either not found in or do not impact on TAVR procedures, which explains the difference with our study.
Radiation exposure from PCI done in one busy cathlab suite at our institution was analysed to give a general comparison with TAVR. It is important to note that the patients were different and the imaging equipment, although from a similar generation, was also different. Hence, comparison between groups is only to give a general sense of the radiation exposure during TAVR. Fluoroscopy time was longer for TAVR but that did not mean greater Ka,r. This could be explained by a lower use of cine during TAVR than during PCI. P KA was however slightly greater for TAVR, possibly explained by the use of a larger x-ray field, especially for TF cases.
For both patients and operators, the risk of a clinically diagnosable cancer is related to the physical dose delivered to each of an individual’s organs, the age variability of organ sensitivity to radiation and that individual’s age and life-expectancy.19-21 Both the International Commission on Radiological Protection (ICRP) and National Council on Radiation Protection and Measurements (NCRP) use effective dose (E) as an estimator of comparative radiation risk for broad radiation protection purposes. Effective dose is estimated by calculating the physical dose delivered to each of the organs in a reference person, applying radiosensitivity weighting factors for each organ, and then calculating the result. Both the ICRP and NCRP state that effective dose is not to be used to describe individual patient risks but is adequate for broad comparisons between procedures. Typically, an anatomically and procedurally-based conversion factor is used to estimate E from kerma-area product (KAP). The factor for adult PCI ranges from 0.14-0.21 and the International Atomic Energy Agency used a factor of 0.18 in a recent report.22 Based on this, E for TAVR is roughly 42 mSv (TF) and 16 mSv (TA). The corresponding E for PCI in the comparison set is 29 mSv. Mettler23 reported nominal E of 0.02 and 0.1 mS for posteroanterior (PA) and PA-lateral chest radiographs, respectively. Comparisons via E must be used with care. This is especially true given the differences between the ICRP person (used to estimate E) and procedural differences between the TA-TAVR, TF-TAVR, PCI, and chest radiographs. A qualitative risk analysis is possible. The “dose” term is proportional to the KAP (PKA) and the irradiated part of the body. Since KAP is similar for PCI and TAVR, per procedure operator cancer risk is likely to be similar for both procedures. In addition, radiogenic cancers do not appear until a latent period ranging from years (leukaemia) to decades (solid tumours). Thus, the risk of clinical cancers attributable to radiation during TAVR is likely to be lower than for PCI due to a two-decade older patient population with potentially more comorbidities.
Several important limitations of the present analysis should be discussed. Although it is the largest published series of radiation exposure during TAVR to date, the number of patients is relatively small and represents the early experience of one academic centre. More liberal use of fluoroscopy at the early stage of this learning process may be expected. However, no reduction in radiation use for TF cases was seen in the second half of treated patients. Calibration drifts of the fluoroscope between semi-annual testing (typically a few percent in the absence of obvious damage to the equipment) are possible.
Despite these limitations, our study shows that the dose of radiation received during TAVR is reasonably low and should not play a major role in the decision-making of the therapeutic approach, especially in elderly patients. Nonetheless, implementation of strategies to decrease radiation exposure should be a priority for all participants involved in procedures requiring radiation use. Reducing radiation use enhances both staff and patient safety.13,15,24-26 Operator exposure (particularly hands) is of greater concern for the TA procedure relative to the TF approach.26 One option to reduce radiation would be to perform valve deployment under fluoroscopy and store the sequence. Also, more significant utilisation of adjunctive modalities such as TEE for positioning of the valve has been shown to reduce contrast volume.27 It is likely that this approach would also reduce the radiation exposure time compared to guidance by fluoroscopy alone.
Conclusion
Radiation exposure during TAVR appears similar to other percutaneous coronary interventions and is within a reasonable range. Patients are unlikely to suffer skin damage as a result of either procedure. Late radiogenic cancers in TAVR patients are unlikely to be due to the combination of patients’ ages and underlying diseases. Radiation doses were significantly lower for TA compared with TF procedures. The higher dose of radiation in TF patients may be related to additional imaging requirements to optimise percutaneous vascular access and closure or to manage complications.
Conflict of interest statement
B. Daneault, S. Kodali, M. Williams and J-M. Paradis have received consultant fees from Edwards Lifesciences. P. Généreux has received speaking honoraria and consultant fees from Edwards Lifesciences. C. R. Smith is a non-paid member of the Scientific Advisory Board of Edwards Lifesciences. M. B. Leon is a non-paid member of the Scientific Advisory Board of Edwards Lifesciences and Medtronic Vascular. The other authors have no conflicts of interest to declare.

