Abstract
Background: Despite improvements in the safety of transcatheter aortic valve implantation (TAVI), ~4% of patients experience a procedure-related stroke. Understanding long-term health and healthcare implications of these events may motivate the development and adoption of preventative strategies.
Aims: We aimed to assess the association of TAVI-related ischaemic stroke with subsequent clinical outcomes and healthcare utilisation.
Methods: We used Medicare fee-for-service claims to identify patients who underwent their first TAVI between January 2012 and December 2017. Previously used ICD-9-CM and ICD-10-CM codes were used to identify TAVI-related ischaemic stroke. Among those with and without TAVI-related ischaemic stroke, we compared the risk of a composite endpoint that included all-cause mortality, acute myocardial infarction, and subsequent stroke using inverse probability treatment weighted Cox regression. We also performed a difference-in-difference analysis to compare 1-year Medicare expenditures and days spent at home during the first year after TAVI.
Results: Among 129,628 primary TAVI patients, 5,549 (4.3%) had a procedure-related stroke. These patients were more likely to be female and have had prior stroke, peripheral vascular disease, ischaemic heart disease, or renal failure. After adjustment, TAVI-related ischaemic stroke was associated with a higher risk of the 1-year composite outcome (HR 1.67, 95% CI: 1.56–1.78), higher 1-year Medicare expenditures (difference $9,245 [standard error 790], p<0.001), and fewer days at home during the first year (difference 16 days [standard error 1], p<0.001).
Conclusions: Among Medicare beneficiaries undergoing TAVI, procedure-related ischaemic stroke was associated with worse outcomes, increased Medicare expenditures, and less time spent at home. Procedure-related ischaemic stroke during TAVI remains a critically important and potentially preventable source of patient mortality, morbidity and healthcare utilisation.
Introduction
Transcatheter aortic valve implantation (TAVI) has transformed the treatment of severe symptomatic aortic stenosis over the last decade, producing similar improvements in survival and quality of life when compared to traditional surgical aortic valve replacement (SAVR)1. This has spurred widespread adoption of TAVI, and in 2019 the annual number of TAVI procedures surpassed that of SAVR2. Despite improvements in TAVI technology and increasing operator proficiency, the risk of clinically-significant stroke after TAVI has remained unchanged at ~4% since 20122. Current strategies to improve the neurological safety of TAVI procedures, such as cerebral embolic protection devices, have been approved by the U.S. Food and Drug Administration (FDA) after a clinical trial showed a trend towards lower brain lesion volume on magnetic resonance scans, although large randomised trials supporting the clinical efficacy and cost-effectiveness of these devices have not yet been completed34. Prior studies have indicated that strokes are associated with increased short-term mortality and cost burden56. However, these studies were limited by their short follow-up, exclusive use of clinical trial data (which limits generalisability), and focus on only in-hospital costs7. Understanding the long-term consequences of TAVI-related ischaemic stroke may help spur the development, evaluation, and adoption of effective strategies to improve the neurological safety of TAVI procedures.
We aimed to assess the association of procedure-related ischaemic stroke with subsequent clinical outcomes and healthcare utilisation among elderly patients undergoing TAVI. In particular, our goal was to quantify the impact of TAVI-related ischaemic stroke on both patient-oriented endpoints and healthcare expenditures, which could help guide shared decision-making and inform the evaluation of strategies designed to reduce procedural stroke.
Methods
Data sources
This analysis was conducted using Medicare Provider Analysis and Review (MedPAR) files and institutional outpatient claims from the Centers for Medicare & Medicaid Services (CMS). The MedPAR database includes information on 100% of claims from inpatient (part A) discharges, skilled nursing facilities (SNFs), inpatient rehabilitation, and long-term acute care facilities (LTAC) for Medicare fee-for-service beneficiaries. The Medicare Institutional Outpatient Database includes information on 100% of claims from outpatient facility charges for Medicare fee-for-service beneficiaries including outpatient expenditures and observation unit or emergency department (ED) visits.
Study population
All Medicare fee-for-service beneficiaries who underwent an initial TAVI procedure between January 2012 and December 2017 were identified for inclusion using previously validated International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and International Classification of Diseases, Tenth Revision, Procedure Coding System (ICD-10-PCS) codes for the TAVI procedure (Supplementary Table 1)8. For patients with repeated TAVIs within the study period, only the first procedure was selected. Patients were excluded if they had a prior SAVR. TAVI-related stroke was defined as an ischaemic stroke occurring during the index hospitalisation for the TAVI procedure. These were identified using previously used ICD-9-CM and International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes for ischaemic stroke (Supplementary Table 1)910. We performed a sensitivity analysis using a validated high specificity, but lower sensitivity, ICD-9-CM and ICD-10-CM algorithm to identify ischaemic stroke11. Prior studies evaluating the timing of these events have shown that the highest risk of TAVI-related stroke occurred at the time of the TAVI procedure6. The study was approved by the institutional review board of Beth Israel Deaconess Medical Center, with a waiver of informed consent.
Patient and public involvement statement
This study utilises an administrative database and no patients were involved in setting the research question or the outcome measures, nor were they involved in the design and implementation of the study. The findings will be disseminated to the public through our research centre social media platforms.
Outcomes
The primary outcome of the study was a composite outcome of the first occurrence of any of the following: all-cause mortality, acute myocardial infarction (AMI, defined using codes in Supplementary Table 1), or subsequent stroke111213. All-cause mortality and AMI were ascertained starting with the index TAVI procedure. Subsequent stroke was defined as an ischaemic stroke occurring after the index hospitalisation. This is because recurrent stroke during the index hospitalisation cannot be differentiated from periprocedural TAVI-related stroke, as these are identified on discharge codes. In addition, the majority of TAVI procedures occurred on day 1 of the hospitalisation; therefore any stroke during the hospitalisation would have occurred after TAVI. For secondary outcomes, we investigated each component of the primary composite outcome individually and 1-year all-cause readmission, defined as any readmission in the year following the index TAVI admission. Deaths were ascertained using vital status information in the Master Beneficiary Summary Files, which was complete for all individuals.
We also investigated health care utilisation using two metrics. First, we evaluated CMS expenditures in dollars by determining the annual Medicare reimbursement, adjusting for inflation using the consumer price index (CPI), with 2018 as the index year14. CMS expenditures included payments for hospital admissions, SNFs, inpatient rehabilitation facilities, LTACs, and outpatient healthcare facilities. Second, we evaluated days at home at 1 year (DAH365). DAH365 is an emerging patient-centred outcome that quantifies the time a patient spends alive and outside of a healthcare facility. Variations of this metric have been studied for several cardiovascular diseases151617. Lower DAH365 indicates higher healthcare utilisation and highly correlates with mortality and functional outcomes after stroke1618. DAH365 was defined as days spent at home, alive, and not in a health care facility, starting with the admission date of the index hospitalisation. We used a comprehensive definition that included inpatient hospitalisations (including the index hospitalisation), inpatient rehabilitation facilities, SNFs, LTACs, ED visits, and observation unit stays. Each ED visit or observation unit stay was counted as 1 day. ED visits or observation unit stays overlapping with a readmission were counted as inpatient days. To calculate time alive, the number of mortality days, defined as the number of days remaining in the year following date of death, was subtracted from DAH365. Thus, DAH365 for each patient was calculated as follows: DAH365=365–(mortality days+inpatient days+inpatient rehabilitation days+SNF days+LTAC days+ED days+observation days)19. All outcomes were analysed up to December 2018.
Statistical analysis
Baseline characteristics of patients with TAVI-related ischaemic stroke were compared with those without TAVI-related ischaemic stroke using Fisher's exact test and t-tests. Kaplan-Meier techniques were used to plot the survival curve of all-cause mortality among patients who underwent TAVI with TAVI-related ischaemic stroke, compared to those without stroke.
Covariates
Patient characteristics included demographics (age, sex, race/ethnicity), dual Medicare-Medicaid enrolment (an indicator of socioeconomic status), and clinical comorbidities. We derived a patient’s clinical comorbidities from two sources: 1) Medicare inpatient data based on the Elixhauser comorbidity algorithm to identify comorbidities during the year prior to hospitalisation, and 2) the Chronic Condition Data Warehouse, which uses a combination of inpatient and outpatient claims to define 67 common chronic conditions2021. Hospital characteristics were identified using the 2018 American Hospital Association survey data, which include hospital teaching status, hospital region, rural/urban status, and bed capacity. Procedural characteristics were evaluated using the MedPAR database and include admission day for TAVI, TAVI access approach, and admission type (emergent, elective). Emergent was defined as an admission that was coded as emergency or urgent in the MedPAR database.
To account for baseline differences between those with TAVI-related ischaemic stroke and those without, we used inverse probability treatment weighting (IPTW) to examine the association between TAVI-related ischaemic stroke and outcomes22. This was performed by calculating propensity scores for each patient using a logistic regression model that included all available covariates to predict the probability of each patient having a TAVI-related ischaemic stroke event. Propensity scores were converted to probability weights that were used to reduce the imbalance in covariates between those with TAVI-related ischaemic stroke and those without. The imbalance of covariates was assessed before and after IPTW adjustment using standardised differences (Supplementary Table 2)23.
Clinical outcomes of TAVI-related ischaemic stroke
In the IPTW-adjusted population, Cox proportional hazards regression was used to compare the risk of the primary composite outcome among those with and without TAVI-related ischaemic stroke. Similar multivariable models, using all listed covariates and year of procedure, were used to compare the risk of the primary outcome and of each individual component of the primary outcome among those with and without TAVI-related ischaemic stroke. To account for the competing risk of death when assessing the individual components of the primary composite outcome, we performed a competing risk analysis, using the method described by Fine and Gray, to estimate the cause-specific hazard ratio for AMI and subsequent stroke24.
Healthcare utilisation outcomes of TAVI-related ischaemic stroke
To evaluate the association between TAVI-related ischaemic stroke and subsequent health care utilisation, we fitted a weighted mixed-effects model using the IPTW approach described above to compare CMS expenditures between those with TAVI-related ischaemic stroke and those without. We also compared the difference in DAH365 between both groups. We then performed a sensitivity analysis, evaluating these relationships using a difference-in-difference analysis in the IPTW-adjusted population. This method controls for time-varying confounders by measuring the outcome of interest before and after the TAVI-related ischaemic stroke25. Since the exact date of TAVI-related ischaemic stroke cannot be determined during the index hospitalisation in Medicare claims, the date of TAVI was used as “time zero” for both comparator groups. The difference in the outcome was then measured between those with a TAVI-related ischaemic stroke and those without, prior to the date of TAVI, and also after TAVI. These two differences were then subtracted to estimate the difference-in-difference. In the evaluation of CMS expenditures, we also used the method described by Bang and Tsiatis to account for the competing risk of death26.
A 2-tailed p-value <0.05 was considered statistically significant and 95% confidence intervals (CI) were calculated for all estimates. All analyses were performed using SAS version 9.4 (SAS Institute).
Results
Baseline characteristics
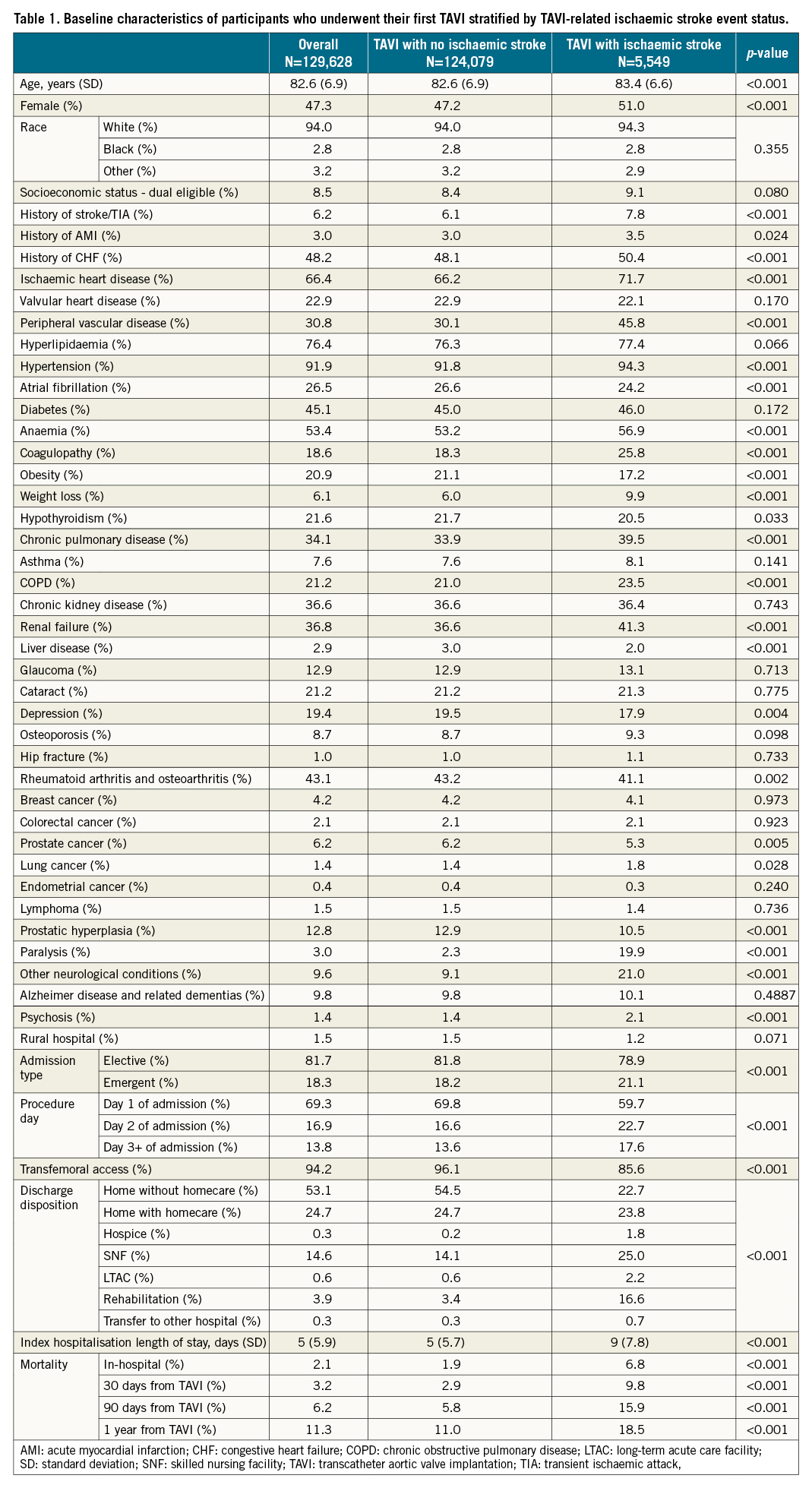
We included 129,628 Medicare fee-for-service beneficiaries who underwent their first TAVI between January 2012 and December 2017 in the analysis, of whom 5,549 (4.3%) had a TAVI-related ischaemic stroke and 124,079 (95.7%) who did not. Patients with a TAVI-related ischaemic stroke were more likely to be female, have ischaemic heart disease, prior ischaemic stroke, congestive heart failure, peripheral vascular disease, hypertension, paralysis and other neurological diseases, chronic pulmonary disease, anaemia, or renal failure (Table 1). Patients with a TAVI-related ischaemic stroke were more likely to have been admitted emergently for their index TAVI procedure, and were more likely to be discharged to an SNF (25% versus 14.1%; p<0.001) or an inpatient rehabilitation facility (16.6% versus 3.4%; p<0.001). Patients without a TAVI-related ischaemic stroke were more likely to have atrial fibrillation and obesity.
Clinical outcomes
Patients with a TAVI-related ischaemic stroke had a higher risk of the primary composite outcome at 1 year (IPTW-adjusted hazard ratio [aHR] 1.67, 95% CI: 1.56-1.78). This was largely driven by an increased 1-year risk of subsequent stroke (aHR 1.99, 95% CI: 1.69-2.34) followed by AMI (aHR 1.66, 95% CI: 1.35-2.06) and all-cause mortality (aHR 1.52, 95% CI: 1.43-1.65) (Table 2). In addition, the associated risk of death was highest at 30 days post-procedure (aHR 3.17, 95% CI: 2.87-3.49). Among those with 5 years of follow-up (TAVI in years 2012-2013), the risk of death persisted (aHR 1.18, 95% CI: 1.10-1.20) (Table 2, Figure 1). Finally, TAVI-related ischaemic stroke was associated with a higher risk of all-cause readmission at 1 year (aHR 1.15, 95% CI: 1.10-1.20). The sensitivity analysis using a validated high specificity ICD algorithm for defining stroke related to TAVI showed similar results (Supplementary Table 3, Supplementary Figure 1).
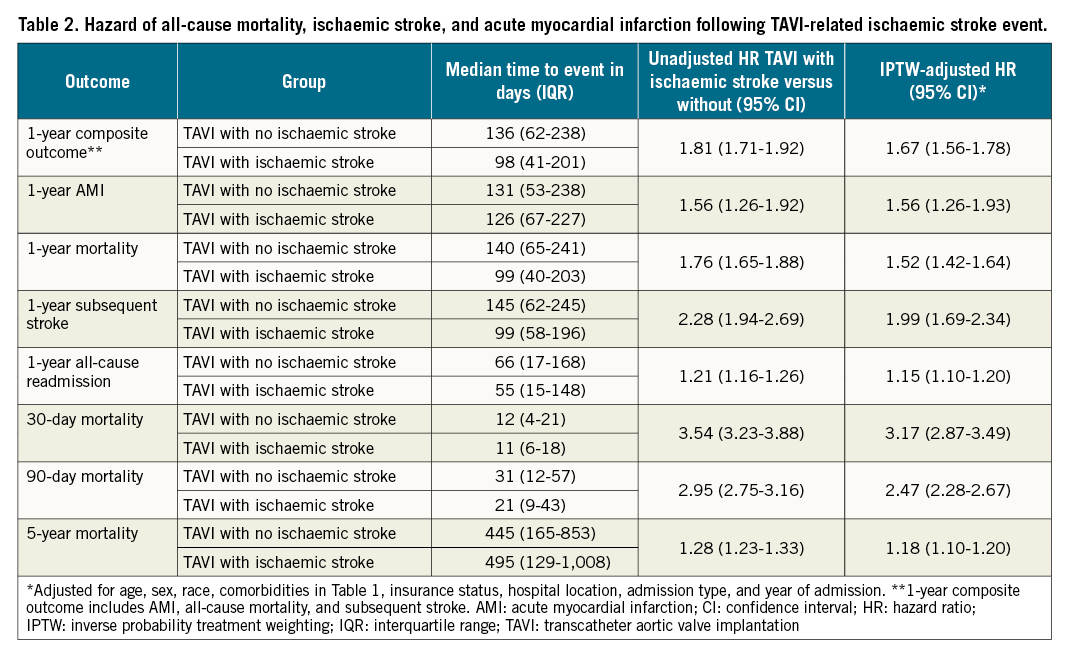
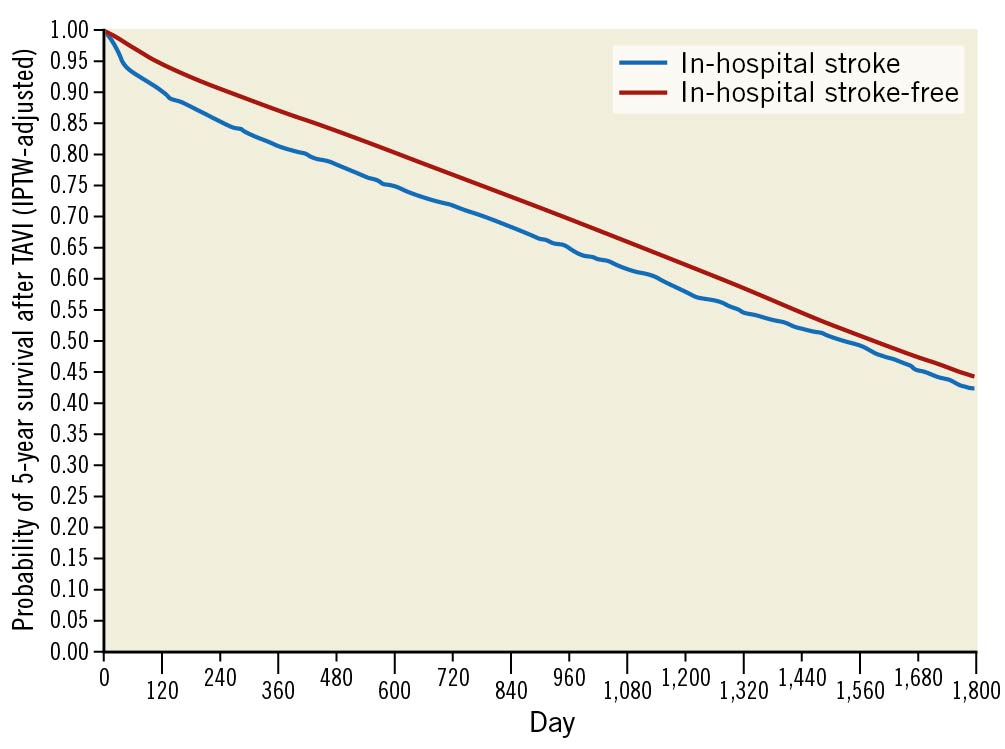
Figure 1. Kaplan-Meier curve showing 5-year mortality in an IPTW-adjusted population. Cumulative survival in patients undergoing TAVI stratified by TAVI-related ischaemic stroke status for entire follow-up period. IPTW: inverse probability treatment weighting; TAVI: transcatheter aortic valve implantation
Healthcare utilisation
After IPTW adjustment, patients with a TAVI-related ischaemic stroke had higher mean 1-year CMS expenditures compared to patients without ($79,373 vs $70,411, p<0.001). This was driven predominantly by the index hospitalisation followed by rehospitalisations, SNF, outpatient healthcare, LTAC, and inpatient rehabilitation facilities (Table 3). Though individuals with a TAVI-related ischaemic stroke had $283 lower CMS expenditures within the year prior to TAVI, they had $8,962 higher CMS expenditure in the year after (p<0.001 for both comparisons). This resulted in a total difference of $9,245 in CMS expenditures (standard error [SE] 790, p<0.001) (Table 4). The additional analysis accounting for the competing risk of death resulted in similar findings (Supplementary Table 4).
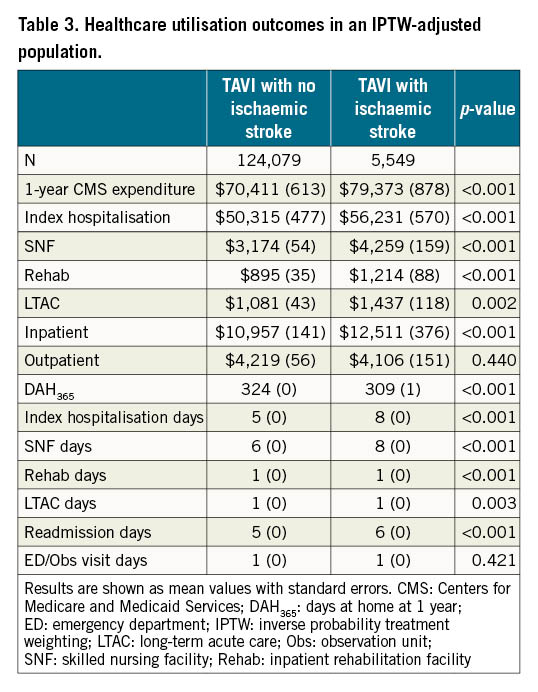
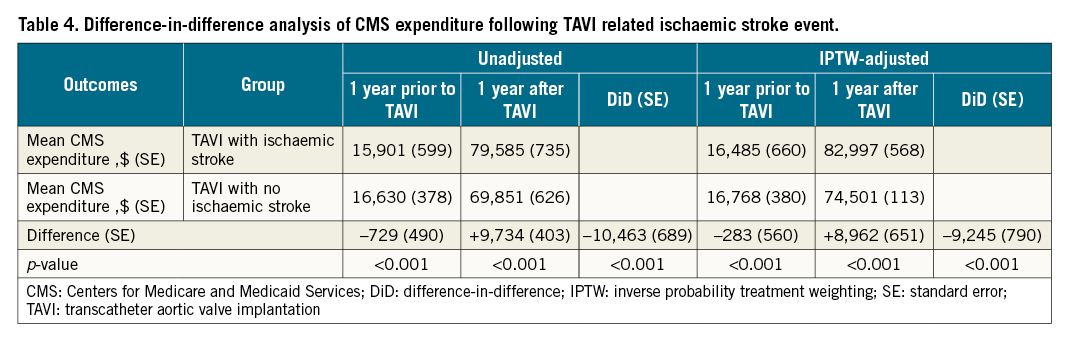
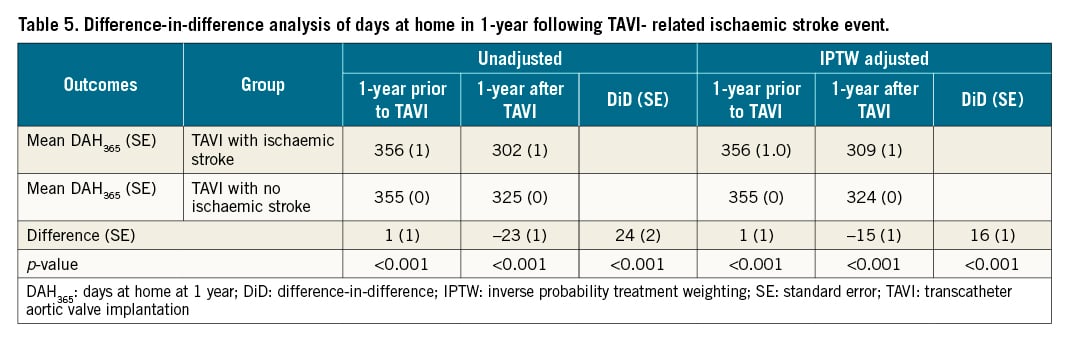
After IPTW adjustment, patients with a TAVI-related ischaemic stroke had fewer mean days at home within the first year (DAH365 309 days versus 324 days, p<0.001). In addition to days lost due to death, the largest facility day contributions were due to additional days spent during index hospitalisation (8 days vs 5 days), SNF (8 days vs 6 days), and readmissions (6 days vs 5 days) (Table 3). Though individuals with a TAVI-related ischaemic stroke had a slightly higher DAH365 in the year prior to TAVI (1 day, SE 1, p<0.001), they had a lower DAH365 in the year after (-15 days, SE 1, p<0.001). This resulted in a total difference of 16 days (SE 1, p<0.001) (Table 5).
Discussion
In this large, nationally representative cohort of patients undergoing TAVI, we found that TAVI-related ischaemic stroke occurred in 4.3% of initial TAVI procedures, and was associated with a higher risk of the composite outcome of all-cause mortality, AMI, and subsequent stroke in the year following the index procedure (Central illustration). Specifically, TAVI-related ischaemic stroke was associated with a higher risk of all-cause mortality that was highest in the first 30 days and persisted for the duration of follow-up. Patients who experienced a TAVI-related ischaemic stroke had higher CMS expenditures ($9,245) and significantly fewer days at home (16 days) in the first year after the index procedure. Overall, these findings help to quantify the significant health, quality of life and economic consequences that result from ischaemic stroke during TAVI, and highlight the strong need to improve our ability to prevent it.
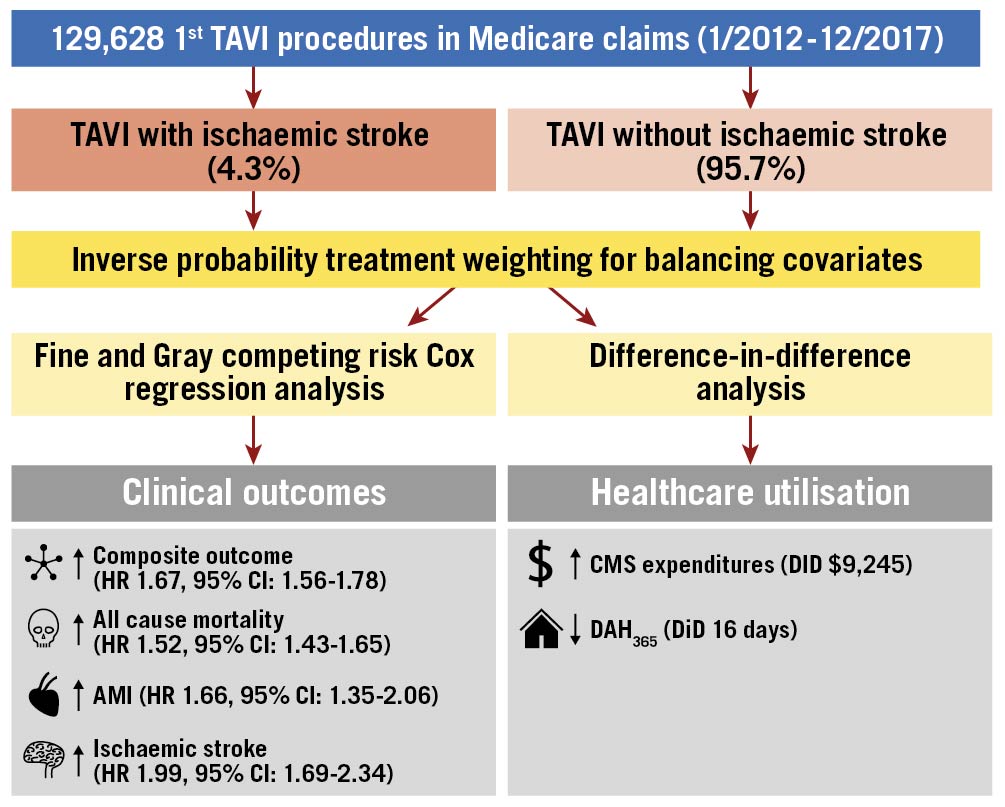
Central Illustration. Clinical and healthcare utilisation outcomes of TAVI-related ischaemic stroke events in a Medicare claims population. Study population, design, and key outcomes. AMI: acute myocardial infarction; CI: confidence interval; CMS: Centers for Medicare and Medicaid Services; DAH365: days at home in 1 year; DiD: difference-in-difference; HR: hazard ratio; TAVI: transcatheter aortic valve implantation
The finding of worse clinical outcomes among individuals with TAVI-related ischaemic stroke is also consistent with prior studies on this subject. In a secondary analysis from the PARTNER (Placement of AoRTic TraNscathetER Valves) trials, TAVI-related stroke was associated with lower 1-year survival in patients undergoing TAVI (47% survival rate for transfemoral TAVI patients with stroke versus 82% for transfemoral TAVI patients without stroke), and a decline in quality of life at 1 year627. A recent study from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) Registry evaluated the short-term outcomes among 101,430 patients who underwent TAVI between November 2011 and May 2017, and showed that TAVI-related stroke was associated with a higher risk of 30-day mortality5. Our work builds on these prior investigations by providing more long-term follow-up and highlights the additional risks of AMI and subsequent stroke.
The finding of increased healthcare utilisation following TAVI-related ischaemic stroke adds to prior studies that were limited in scope. In a study from the National Readmission Database of ~30,000 patients who underwent TAVI between January 2013 and December 2014, the authors evaluated 30-day outcomes of TAVI-related stroke. Similar to our findings, these events were associated with higher cost, increased length of stay, and higher incidence of non-home discharges28. In another study from the PARTNER 1 trial, TAVI-related stroke was also associated with increased cost ($15,231) and length of stay (2.5 days), after adjusting for patient characteristics29. However, compared to our study, these studies were limited to early TAVI experiences, had limited follow-up, and did not include non-hospital costs. In a study from the STS/ACC TVT Registry, Vemulapalli et al evaluated healthcare utilisation associated with TAVI using a before and after design (1 year before and 1 year after)30. The authors noted increased healthcare utilisation following TAVI, as evident by an increase in all-cause hospitalisation. In addition, the mean 1-year CMS expenditures post-TAVI were $75,585±$41,135. This was $7,412 lower than our findings for TAVI-related stroke and $1,084 higher than our findings for TAVI with no stroke. In addition, our CMS expenditure estimates included a broader range of facilities as they did not include expenditures related to inpatient rehabilitation or outpatient facilities.
Although DAH365 has been used to examine outcomes after ischaemic stroke, to our knowledge this is the first application of this novel patient-oriented metric to TAVI-related stroke. In a study from the Get With The Guidelines-Stroke (GWTG-Stroke) and Adherence eValuation After Ischemic Stroke-Longitudinal (AVAIL) Registries, 815 patients with ischaemic stroke were noted to have 349 (interquartile range [IQR] 303-360) median days at home when counting inpatient, SNF, and rehabilitation days only16. Days at home in the medical stroke population were higher than what we observed in patients undergoing TAVI (349 days vs 324 days). This is likely due to our inclusion of a more comprehensive definition of DAH365, as their definition excluded home days lost due to index hospitalisation, index hospital death, LTAC, ED visits, and observation unit stays. It is also unclear how the severity of stroke differs between the two populations. Overall, these findings of higher healthcare utilisation highlight the toll of TAVI-related stroke on patients and the healthcare system.
There are several strengths to this study. First, it includes a large national cohort of patients undergoing their first TAVI procedure in the United States. Second, it is the first study providing long-term follow-up on TAVI-related ischaemic stroke. Third, the quasi-experimental design used in this analysis reduces the risk of unmeasured confounders and helps make causal inferences. Finally, the study uses an important patient-centred outcome in using DAH365, which has a more comprehensive definition than those used in prior studies.
Limitations
The study also has several limitations. First, despite the use of contemporary quasi-experimental study design, the study is observational and carries the risk of residual confounding. We attempted to address these concerns by using both propensity score-based inverse weighting, using a large number of clinical covariates, and difference-in-difference sensitivity analyses, which may be more resilient to unmeasured confounding. Second, the study only included Medicare beneficiaries, limiting the generalisability to younger patients. Third, we did not include patients enrolled in Medicare Advantage plans, as we are unable to accurately capture the outcomes of interest in this population. These patients represent 26-34% of all Medicare enrollees during the time of the study31. Fourth, given the expanding indications for TAVI in low-risk patients, ischaemic stroke rates might have decreased in recent times and therefore this analysis only reflects the experience of TAVI during the years included in the study. Fifth, Medicare expenditures used in this study may not reflect true comprehensive costs as this study mainly focuses on inpatient and outpatient facility-related costs and does not include indirect health care costs (e.g., out-of-pocket costs, productivity loss). Finally, our study defined TAVI-related ischaemic strokes as those occurring in the index hospitalisation, compared with the 30-day eligibility defined in the recent Valve Academic Research Consortium 3 report, in an attempt to limit the risk of immortal time bias32. However, prior studies have shown that the highest risk of TAVI-related ischaemic stroke is at the time of the procedure6.
Conclusions
In conclusion, among Medicare beneficiaries, TAVI-related ischaemic stroke was associated with significant increases in subsequent major cardiovascular events, a decline in days spent at home, and an increase in healthcare utilisation in the year following the index procedure. These findings clarify the health and economic consequences of TAVI-related ischaemic stroke, and inform the evaluation of new strategies and devices intended to prevent them.
Impact on daily practice
Among Medicare beneficiaries undergoing TAVI, TAVI-related ischaemic stroke occurred in 4.3% and was associated with a higher risk of death, acute myocardial infarction, and subsequent stroke. In addition, TAVI-related ischaemic stroke was associated with higher healthcare utilisation, with higher 1-year Medicare expenditures and fewer days at home at 1 year. These findings highlight TAVI-related ischaemic stroke as a critically important and potentially preventable source of patient morbidity. In addition, it informs clinicians, patients, and health systems on the health and economic consequences of TAVI-related ischaemic stroke that will guide the evaluation of new strategies and devices intended to prevent them.
Funding
This work was funded by the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology.
Conflict of interest statement
Z. Almarzooq received research support from the Kuwait Foundation for the Advancement of Sciences (KFAS). M. Chung is funded by the National Institute of Health (T32-GM007592) and by Medtronic, outside the submitted work. R. Yeh receives research support from the National Heart, Lung, and Blood Institute (R01HL136708), the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology and Philips Medical; he reports consulting fees from Abbott Vascular, Boston Scientific and Medtronic. D.S. Kazi declares institution grants from National Institutes of Health/National Heart, Lung, and Blood Institute, Bethesda, MD, USA and from the Harvard Global Health Institute, Boston, MA, USA. He also receives institution and individual grants from the Institute for Clinical and Economic Review, Boston, MA, USA. He has a voluntary role, with no reimbursement except for travel expenses for meetings, with the American Heart Association, Dallas, TX (Science Chair, International Committee). J.B. Strom declares funding from NIH/NHLBI, grants from Edwards Lifesciences, Ultromics, HeartSciences and Anumana; consulting fees from Bracco Diagnostics; honoraria from Northwest Imaging Forums. He serves on the Board of Directors of the American Society of Echocardiography and on the Joint Review Committee on Diagnostic Medical Sonography; and has a role in the International Contrast Underground Society. S. Baron declares receiving institution grants from Boston Scientific and Abiomed; personal consulting fees from MitraLabs, Abbott and Edwards Lifesciences; honoraria from Boston Scientific; receives advisory board fees from Boston Scientific and Abiomed; she is also part of the SCAI Women in Innovations Committee. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

