Abstract
In recent years, transcatheter aortic valve implantation (TAVI) has been demonstrated to be at least as effective as surgical aortic valve replacement in high-risk patients with severe symptomatic aortic stenosis. Today, the procedure is highly standardised and reproducible, and one of the most debated issues is whether TAVI is ready to be simplified. In experienced centres, a simplified or minimalist approach to TAVI is already routine and it has been shown to be as safe and effective as the more traditional approach in selected patients.
Introduction
Transcatheter aortic valve implantation (TAVI) has rapidly evolved as a viable alternative to surgical aortic valve replacement in patients with severe symptomatic aortic stenosis who are at high surgical risk or deemed inoperable1. During its short history, the results and impact of this procedure on clinical practice have already gone way beyond initial expectations. In fact, after the first pioneering experiences, research in the TAVI field has shifted quickly - exploring concepts such as “feasibility” at the very early stage, moving towards “effectiveness”, and lately “simplification” and “optimisation”.
Today, the procedure is highly standardised and reproducible with extremely favourable clinical success rates, which are expected to improve further with the development of new TAVI devices and increasing operator experience. Therefore, while we await the results of very long-term follow-up (up to 10-15 years) to assess the durability of the benefit so far reported, one of the most debated issues is whether TAVI is ready to be simplified.
In the vast majority of centres, TAVI is still considered a very complex procedure for many reasons: i) the population treated is usually elderly with several comorbidities; ii) TAVI requires significant technical expertise and a multidisciplinary “Heart Team” to optimise patient screening and case selection; iii) the procedure is often performed under general anaesthesia; iv) post-procedural hospitalisation is generally quite prolonged; and, last but not least, v) the procedure and devices are expensive.
However, in recent years some North American and European groups (including ours) have been working on local programmes which incorporate specific pre-, peri- and post-procedural pathways aimed at simplification of the TAVI pathway2-4. These approaches have potential to optimise pre-procedural screening, dramatically reduce the invasiveness of the procedure, and shorten the duration of hospitalisation, thus saving resources and accelerating the functional recovery of the patient. However, the objective of saving resources has to be achieved without compromising procedural safety in order for a minimalist approach to TAVI to be termed “sustainable” and “economically attractive”.
Pre-procedural screening (Figure 1)
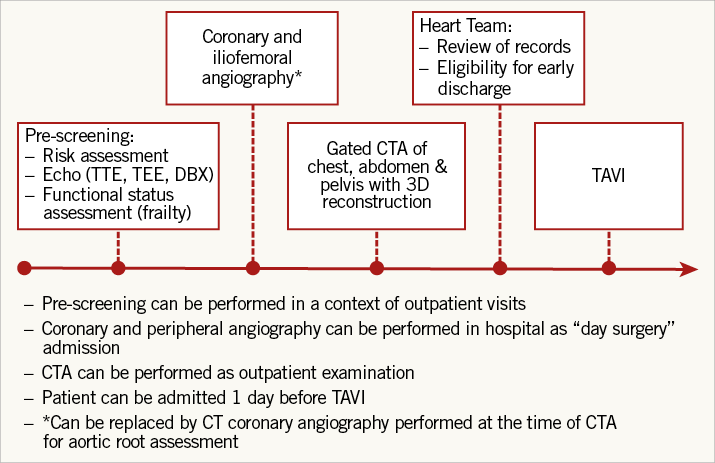
Figure 1. Example of optimised patient flow for TAVI screening.
The local Heart Team should screen all patients with severe aortic stenosis who are high-risk candidates for aortic valve replacement. Once the diagnosis and indication for TAVI are confirmed, patients should undergo multi-detector computed tomography (MDCT) to facilitate pre-procedural planning, valve sizing and prosthesis selection. MDCT is relatively contraindicated in patients with impaired renal function (a frequent comorbidity in TAVI patients), and transoesophageal echocardiography (TEE) with three-dimensional reconstruction of the aortic root is a useful alternative.
Screening for coronary artery disease is also necessary before TAVI. Although invasive coronary angiography remains the gold standard for this assessment, Chieffo and colleagues recently demonstrated that CT coronary angiography can be performed safely and effectively as a routine non-invasive imaging tool in TAVI patients, allowing acquisition of information concerning aortic annular anatomy, peripheral access sites and coronary anatomy with a single investigation5.
Procedure
There are some procedural expedients that can be applied to optimise the TAVI procedure itself. A fully percutaneous transfemoral approach should be chosen as the default strategy. Subject to evaluation by an anaesthesiologist, a local anaesthesia/conscious sedation protocol is preferred to reduce the risk of periprocedural hypotension and agitation in the older TAVI population. General anaesthesia should be reserved for selected cases and patients extubated immediately after the procedure. This strategy is supported by a recent meta-analysis in which local anaesthesia was associated with a shorter TAVI procedure, earlier hospital discharge and similar short-term clinical outcomes compared with general anaesthesia6. Although general anaesthesia is usually required to facilitate intraprocedural TEE, TAVI is performed in many centres using fluoroscopy and transthoracic echocardiography only, with excellent results7.
Direct valve implantation (without preliminary balloon dilatation) is feasible in most patients using second-generation TAVI devices and is routinely applied in many centres.
After large sheath removal at the end of the procedure, haemostasis at the iliofemoral puncture site should be achieved with closure devices followed by check angiography via the contralateral side (which also allows rapid access for balloons and stents in the event of unexpected complications).
In the absence of significant acute periprocedural conduction disturbances (severe symptomatic bradycardia, third degree atrioventricular block), the temporary pacing wire can be removed in the catheterisation laboratory or operating room at the end of the procedure. If prolonged temporary pacing is required, then a jugular approach and balloon-tipped pacing wire are recommended to allow early patient mobilisation.
Post-procedural care
Immediately after the procedure, all patients must be monitored in the catheterisation laboratory or operating room for at least 10-15 minutes with special attention to haemodynamics and cardiac rhythm. Thereafter, patients should be transferred to a coronary care (CCU), high care or intensive care unit (ICU), according to local protocols. The overall CCU/ICU length of stay should be no more than 24 hours. After 8-18 hours, the Heart Team should re-assess the clinical status of the patient with particular attention to procedural outcome, ECG, echocardiographic and laboratory findings. In the absence of complications, the patient can be transferred to the cardiology ward after 12-24 hours and commence mobilisation immediately. Permanent pacemaker implantation should be considered for persistent intraventricular conduction delay or symptomatic bradycardia, and central venous and urinary catheters (if used) should be removed as early as is safe and feasible.
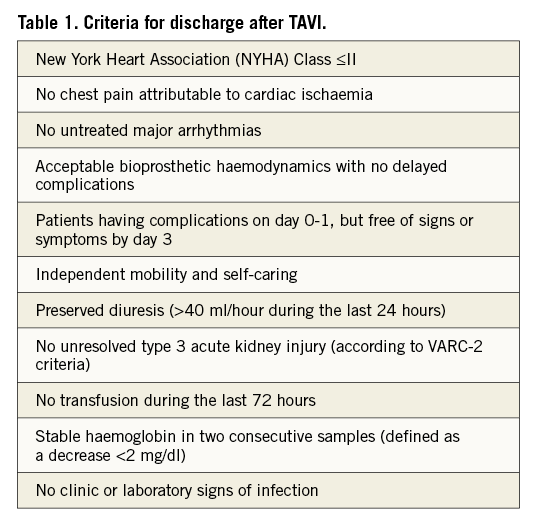
Criteria for discharge in our institution are listed in Table 1. Following early discharge, we recommend repeat outpatient blood tests (including full blood count, renal function and electrolytes) at day 5-7 after discharge in order to rule out significant anaemia or acute kidney injury. The motivation to reduce the duration of hospitalisation after TAVI stems from the desire to accelerate recovery and mobilisation, and minimise unnecessary use of resources. Indeed, the high cost of TAVI is emerging as one of the main limitations for the diffusion of this technique.
Conclusions
Although TAVI is a complex procedure, important steps towards simplification of the procedure have already been made. In experienced centres, a simplified or minimalist approach to TAVI is already routine and has been shown to be as safe and effective as the more traditional approach in selected patients. In our view, it is probably too early to define TAVI as a PCI-like procedure, but it looks like this is in the pipeline for the near future.
Conflict of interest statement
M. Barbanti is a consultant for Edwards Lifesciences. C. Tamburino has no conflicts of interest to declare.

