
The pioneering first-in-human transcatheter aortic valve implantation (TAVI), performed by Cribier in Rouen in 2002, was a turning point in the management of high operative risk patients with severe aortic stenosis1. Was one of the key aspects from this case overlooked during the early TAVI era? Cribier – a cardiologist – performed this procedure in the cath lab with local anaesthesia (LA) and sedation. Subsequently, it was the surgical management of vascular access that propelled the use of general anaesthesia (GA) and a more intensive procedural approach rather than the valve implant itself2. Have we overcomplicated TAVI in the years that followed?
A multidisciplinary Heart Team approach is pivotal to the management of patients with aortic stenosis and complies with guideline recommendations (Class 1)3. The standardised use of multislice computed tomography (MSCT) and dedicated CT analysis software has transformed procedural planning for TAVI. The optimal access route, device selection and the TAVI procedural team can be planned well in advance of the actual procedure and should be intentionally adapted to the specific characteristics of the individual patient4. MSCT has removed the need for routine procedural transoesophageal echocardiography (TOE) and the accompanying GA. GA, previously routine practice, has been gradually replaced by LA +/- conscious sedation in recent years, a trend reflected in national TAVI registries around the world, e.g., the France TAVI registry reported a 30 to 70% increase in LA TAVI from 2010 to 20175. LA is not associated with increased mortality, conversion to sternotomy, or major procedural complications and has the benefits of shorter inpatient stays and a decreased incidence of “low-output” syndromes5-7.
TAVI-specific wires, improved sheaths, even better valves, and techniques such as pacing via the LV wire all have the potential to reduce further the major complications that may require a surgeon and anaesthetist for their management.
With a maturing TAVI landscape and a wealth of data to guide our decisions, simplification of the TAVI procedure has occurred in many centres. Naturally this has raised questions as to who is actually required in the procedure room for optimised TAVI.
In this issue of EuroIntervention, Droppa et al8 describe an interesting comparison they were able to make between patients treated with a “minimalist” Heart Team attending for the TAVI procedure, and subsequent patients treated with a “complete” Heart Team attending for the TAVI.
This established TAVI centre took the opportunity to conduct this study following mandatory changes in German national guidelines for TAVI which came into force in 20158. Although an observational study (conducted over a period between 2014 and 2017), they describe, in considerable detail, the two different approaches applied to consecutive patients attending one centre for TAVI using local anaesthesia. This gives us a unique opportunity to assess the clinical benefits or drawbacks of the specific Heart Team present in the procedure room for the TAVI.
Current guidelines still advocate that TAVI is performed in centres with on-site cardiac surgery (CS). The cardiac surgeon is a central member of the Heart Team, but does this mean that they must always be present in the procedure room at the time of TAVI? The answer, provided by Droppa et al8 in this issue, is no. They found no significant differences in periprocedural and in-hospital outcomes for the “minimalist” versus the “complete” procedural teams.
Droppa et al8 report a 0.3% conversion to open heart surgery for their “minimalist” Heart Team compared to 0.8% for the “complete” Heart Team, supporting the acceptability and safety of the minimalist approach.
From the inception of our own TAVI programme in 2008, our default approach has been to perform TAVI under LA by a percutaneous transfemoral (TF) route, following Heart Team evaluation. To date, 92% of patients have had LA-TF TAVI, 3.1% via LA-alternative access and 4.9% under GA-TF or GA-alternative access. Our conversion rate to GA with sternotomy is 0.6%, due to left ventricular wire perforation (0.5%) and annular disruption (0.1%). We believe we should be able to virtually eliminate wire perforation with a combination of procedural vigilance, the use of dedicated TAVI wires and improvements in device delivery.
Guidelines have now extended indications for TAVI to intermediate-risk patients, based on the outcomes of major trials such as SURTAVI and PARTNER 2A, while the studies of low-risk patients randomised to either TAVI or surgical aortic valve implantation are both recruiting and reporting imminently3,9-11.
Like Droppa et al8, we do not advocate TF TAVI being performed in centres that do not offer CS and, while a cardiac surgeon does not need to be present at the time of most TAVI procedures, they should be readily available for patients in need of CS intervention.
Pathways to access teams that are required to manage TAVI complications should be protocolised and easily activated by cath lab staff. The activating and responding teams should be involved in the design of these protocols and their implementation. They should be embedded in hospital training and ideally be refreshed and tested with simulation at regular intervals. These need to be country and institution appropriate. For example, in the UK, few cardiac surgeons formally train in vascular surgery, so in our hospital a vascular surgeon is an important member of the TAVI team, as major vascular complications are still reported in up to 5% of patients6. The access options for TAVI continue to expand, with transapical, direct aortic, subclavian/axillary, transcarotid, transcaval and the original transseptal anterograde approaches all possibilities. A selected combination of cardiologist, cardiac surgeon, vascular surgeon and interventional radiologist may be appropriate to execute these procedures successfully.
In practice, what does an optimised TAVI pathway look like? The key aspects are summarised in Figure 1.
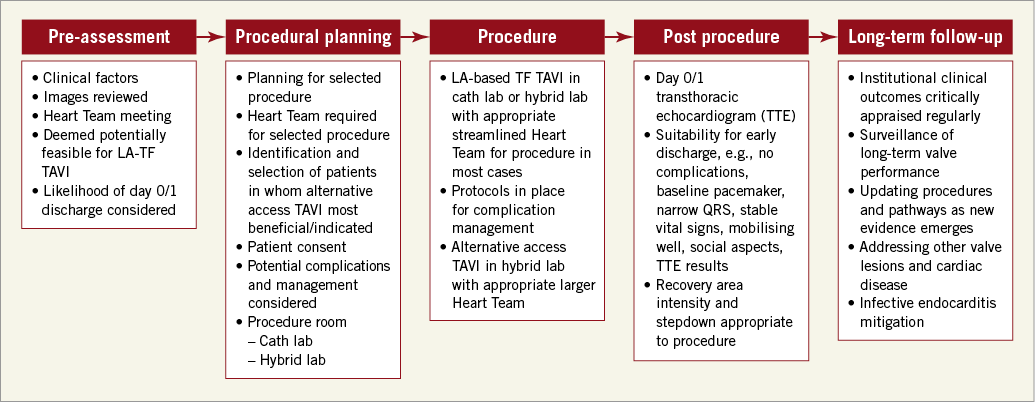
Figure 1. An optimised TAVI pathway.
This optimised pathway must be managed by the Heart Team for the patient. An appropriate streamlined TAVI procedural team needs to be selected and deployed according to the specific characteristics of each patient. In our centre, similar to Droppa et al8 for a “minimalist” Heart Team TAVI, we have two TAVI operators, a nursing team (scrub nurse, circulating nurse, valve loader), a radiographer and a cardiac physiologist present for routine percutaneous TF TAVI, using LA, extending and modifying the team where indicated. We have protocolised rapid activation of cardiac surgery, vascular surgeons, interventional radiology, stroke physicians and an interventional neuroradiologist in the event of complications. While these specialties are readily available, procedural self-reliance demands a focus and diligence which we believe drives excellence.
Alternatively, for our patients who do not undergo routine LA-TF TAVI, we select a procedural Heart Team consisting of a cardiac and/or vascular surgeon, interventional cardiologist, anaesthetist, nurses (cardiac surgical theatre, cath lab nurses and loader) and perfusionist. These cases are performed in the same hybrid cath lab as our routine TAVI procedures, with a cardiopulmonary bypass machine primed and in the room. These patients follow a fast track extubation pathway in our post-op CS intensive care unit.
Regardless of the procedural team, it is absolutely essential that we place the patient at the centre of the Heart Team discussions, ensuring that they give informed consent regarding the nature of the intervention and potential complications. For some patients with limited physiological reserve but still considered appropriate for TAVI, it is important that we openly acknowledge in advance of the procedure the potential futility of salvage interventions. In this context, a major complication, while infrequent, is often terminal. Informed consent may therefore sometimes appropriately include a plan for no attempted surgical salvage.
The Heart Team should be patient-centred and focused on delivering optimised individualised care (Figure 2).
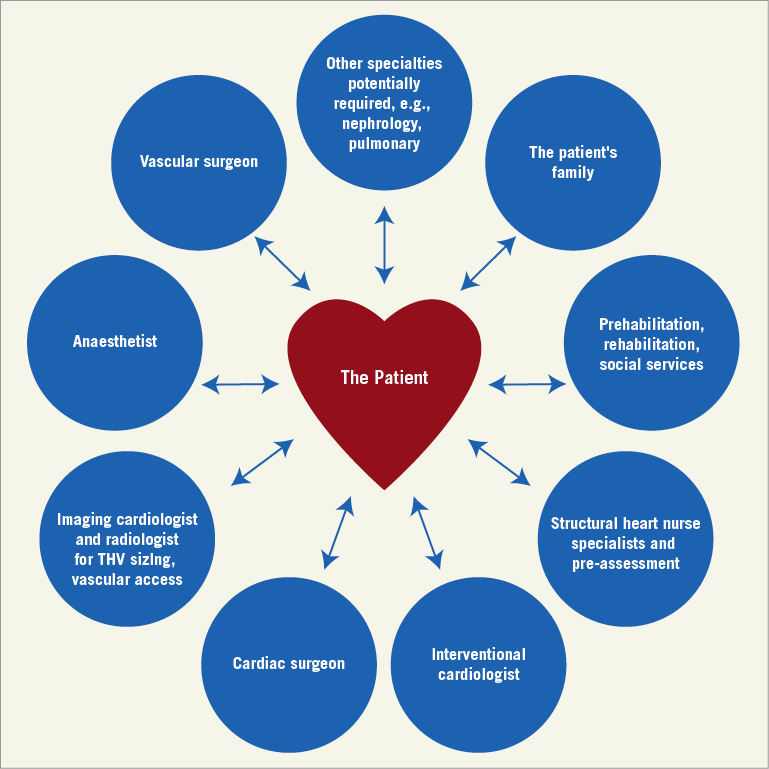
Figure 2. Patient-centred TAVI Heart Team.
An optimised and streamlined TAVI programme leads to greater efficiency, better use of resources and decreased patient stay, which has been associated with decreased post-procedural complications such as delirium12. With the continuing growth in patient referral numbers and the expanding evidence base, it is imperative that TAVI centres are data-driven and critically appraise outcomes, modifying their approach accordingly. TAVI is a fast-moving field and we can never be complacent. Correct patient selection will identify those patients most likely to benefit from a streamlined approach. The FAST-TAVI trial recently reported the safe early discharge (within 72 hours) of over 70% of unselected “all-comer” TF TAVI patients, highlighting the progress and evolution of TAVI over the last decade13.
Conclusion
The first in-human TAVI showed us that it is possible to perform TAVI as a minimally invasive procedure. It is understandable that the early TAVI era then saw large Heart Teams present during the TAVI procedure as it became an established treatment. Now, simplified TF TAVI can be offered safely to most patients with low procedural complication rates, short hospital stays and excellent clinical outcomes. We contend that the complete Heart Team is vital for the management of patients with aortic stenosis and should thoughtfully configure the procedural team to meet the specific needs and challenges of each individual patient efficiently and effectively. This allows optimised procedural outcomes and patient experience. We may need to reshape our approach as the low-risk TAVI trials begin to report; however, this interesting paper provides supportive evidence that we may not need to deviate too far from our current path8.
Conflict of interest statement
M. Spence reports being a TAVI proctor for and receiving honoraria from Medtronic, Edwards Lifesciences and Boston Scientific, and receiving honoraria from Abbott. The other authors have no conflicts of interest to declare.

