
Abstract
Aims: The aim of the study was to evaluate peri-interventional complications and in-hospital complications in different team settings when performing transfemoral aortic valve replacement (TAVR) under local anaesthesia.
Methods and results: We performed TAVR under local anaesthesia with a minimalist Heart Team consisting of two interventional cardiologists, an echocardiographer and two cardiac catheterisation laboratory nurses. In August 2015, new guidelines for TAVR were issued by the National Federal Joint Committee. In accordance with these guidelines, we began to perform TAVR using a complete Heart Team, consisting of two interventional cardiologists, an echocardiographer, a cardiac surgeon, an anaesthesiologist, a cardiovascular perfusionist, two cardiac catheterisation laboratory nurses, a surgical nurse and an anaesthetist nurse. In this study, we retrospectively analysed periprocedural and in-hospital outcomes. Two hundred and ninety-two (55.1%) patients were treated by the minimalist Heart Team, whereas 238 (44.9%) were treated by the complete Heart Team. There were no significant differences in periprocedural (1.4% vs. 1.3%, p=1.0) and in-hospital mortality (4.8% vs. 5.0%, p=0.9) as well as in conversion to open heart (0.3% vs. 0.8%, p=0.59) or immediate vascular surgery (0.3% vs. 2.1%, p=0.1) for minimalist versus complete Heart Team, respectively.
Conclusions: TAVR under local anaesthesia can be safely performed by a minimalist Heart Team. We did not observe any differences in fatal periprocedural complications and mortality when compared with those of a complete Heart Team.
Abbreviations
ACE: angiotensin-converting enzyme
ECLS: extracorporeal life support
G-BA: Gemeinsame Bundesausschuss (the Federal Joint Committee)
GFR: glomerular filtration rate
ICU: intensive care unit
LVEF: left ventricular ejection fraction
VARC-2: Valve Academic Research Consortium-2
Introduction
Transfemoral aortic valve replacement (TAVR) is a standard therapy for aortic valve stenosis in patients at intermediate to high risk for conventional surgery1,2. Performing this procedure under local anaesthesia, compared to general anaesthesia, has been shown to be safe and effective3-6. However, the exact perioperative setting concerning the procedure team set-up varies according to local experience and operator preference. Although the traditional procedure is usually performed with the presence of a thoracic surgeon and an anaesthesiologist, cases which necessitate conversion to open heart surgery are rare. As TAVR can be handled well by an interventional cardiologist and as the experience of operators grows, more centres are now performing the procedure using a minimalist approach under local anaesthesia and omitting the presence of a surgeon and anaesthesiologist. This practice could be resource saving; however, it brings possible risks of delaying surgical management in cases of possible severe periprocedural complications with a risk of higher adverse events. Due to safety concerns, some national regulations were introduced to guarantee a high quality of care. For instance, the Federal Joint Committee (G-BA) in Germany introduced new national mandatory guidelines for TAVR procedures which came into force in August 20157. According to the guidelines, all TAVR procedures have to be performed with a thoracic surgeon and anaesthesiologist in attendance in order to guarantee patient safety and a high quality of care. So far, however, no studies have been performed to compare the influence of a minimalist team setting for TAVR procedures on perioperative risk. Thus, we retrospectively evaluated periprocedural and in-hospital complications in different team settings when performing TAVR.
Methods
We retrospectively analysed all consecutive TAVR procedures performed under local anaesthesia from February 2014 to May 2017 at the University Hospital of Tübingen. An interdisciplinary Heart Team consisting of interventional cardiologists, cardiac surgeons and anaesthesiologists made the decision for treatment by TAVR based on perioperative risk assessment. The EuroSCORE as well as other relevant risk factors not included in the EuroSCORE were used for risk stratification. Over recent years, together with rising expertise, performing TAVR under local anaesthesia by a minimalist Heart Team has been established in our clinic, as previously described8,9. After national guidelines came into force in August 2015, we were forced to change this practice. According to these mandatory guidelines, complete Heart Teams must be present in the operating room during the whole TAVR procedure. Thus, we changed the setting and performed TAVR with a complete Heart Team (Figure 1). Team settings were as follows and as shown in Table 1.
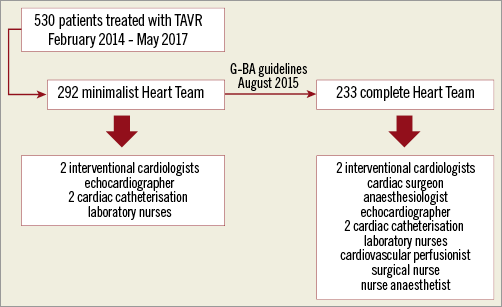
Figure 1. Study flow chart.
MINIMALIST HEART TEAM
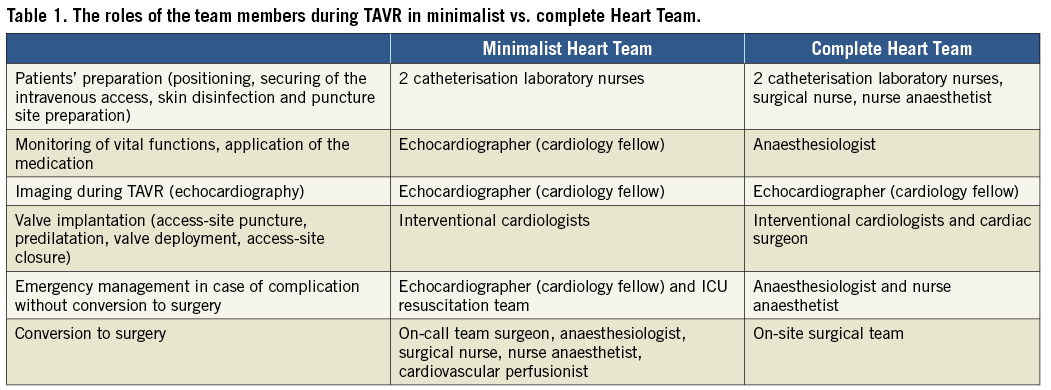
The team consisted of two interventional cardiologists, an echocardiographer (cardiology fellow) and two cardiac catheterisation laboratory nurses. The preparation of patients including positioning, securing of the intravenous access, skin disinfection and puncture site preparation was performed by cath lab nurses. TAVR was performed by the interventional cardiologists without the attendance of a cardiac surgeon. The perioperative monitoring of vital functions, intravenous medication application and first emergency management until the arrival of a resuscitation team in case of complications was the responsibility of the cardiology fellow/echocardiographer, who had previous intensive care unit (ICU) experience. A resuscitation team provided by our ICU was available within one to two minutes after an emergency call in case of severe complications not necessitating conversion to surgery. Surgical teams consisting of a cardiac surgeon, an anaesthesiologist, a cardiovascular perfusionist, a surgical nurse and a nurse anaesthetist were available in the clinic with short arrival times (within about five minutes) in case of complications necessitating conversion to surgery. For severe complications we have extracorporeal life support (ECLS) available on site which could be implanted immediately by an interventional cardiologist while waiting for the arrival of the surgical team.
COMPLETE HEART TEAM
The team consisted of two interventional cardiologists, an echocardiographer, a cardiac surgeon, an anaesthesiologist, a cardiovascular perfusionist, two cardiac catheterisation laboratory nurses, a surgical nurse and a nurse anaesthetist. Preparation of the patient was performed by the cath lab team, surgical and anaesthetist nurses. An anaesthesiologist was responsible for securing the intravenous access, application of intravenous medication, analgesia, potential mild sedation and patient monitoring during the procedure. The valve was implanted by a team of interventional cardiologists and a cardiac surgeon. All of them were, in an alternating manner, involved in the valve implantation process including access-site preparation, valve predilatation and valve deployment. A cardiovascular perfusionist was present as “stand-by” in case of conversion to open heart surgery.
DESCRIPTION OF THE TAVR PROCEDURE
Preoperative diagnostic work-up with transthoracic echocardiography to assess the valve anatomy, the degree of calcification, concomitant valve disease and ventricular function was performed in all patients. The severity of the aortic stenosis was measured by Doppler echocardiography with assessment of transvalvular gradients, flow status and aortic valve area. If necessary, transoesophageal echocardiography was performed for structural analysis and procedure planning. In some cases, dobutamine stress echocardiography was performed to differentiate pseudo-severe aortic stenosis from low-flow low-gradient aortic stenosis2. All patients underwent coronary angiography to screen for concomitant coronary artery disease. High-grade coronary stenoses were treated with percutaneous coronary intervention before TAVR. Computed tomography (CT) angiography for annulus sizing and evaluation of the access site was performed in almost all patients with the exception of patients with severe renal impairment. Based on CT angiography, aortic annulus sizing for appropriate prosthesis selection was performed using dedicated software (3mensio; Pie Medical Imaging, Maastricht, the Netherlands). The distance of the coronary ostia from the annulus was assessed to screen for the potential risk of coronary obstruction. CT angiography of the whole aorta and pelvic vessels was used to evaluate the eligibility for transfemoral valve delivery. All procedures were performed through transfemoral access. Perioperative monitoring was achieved with a 3-channel electrocardiograph (ECG), blood oxygen saturation and continuous blood pressure monitoring through the femoral access. Implantations were performed under local anaesthesia with the addition of intravenous analgesia and/or mild conscious sedation if needed. A percutaneous suture-mediated closure system (either two ProGlide® or one Prostar® XL; Abbott Vascular, Santa Clara, CA, USA) was placed before the delivery of the introducer sheath. Subsequently the TAVR procedure was conducted. Balloon predilatation was performed at the discretion of the operator depending on the severity of calcification and according to the instructions for use for the prosthesis type. Balloon-expandable valves were implanted under rapid ventricular pacing (with 200-220 beats/minute) through a pacing catheter placed in the right ventricle. For the self-expanding valves fast pacing was used if necessary. The position of the prosthesis and any possible paravalvular leak were assessed by angiography and transthoracic echocardiography. After sheath removal, the access site was closed by the closure system and controlled by contralateral contrast injection to rule out bleeding and vascular complications.
In this study, we retrospectively analysed the periprocedural and in-hospital outcome of patients comparing minimalist and complete Heart Team settings. Patient characteristics, perioperative and postoperative course and complications, including intrahospital mortality, were recorded from patients’ files. The pre-specified endpoints of the analysis were immediate periprocedural mortality (defined as intraprocedural events resulting in immediate or consequent death within 72 hours according to VARC-2)10, in-hospital mortality, conversion to open heart surgery and major vascular complications requiring surgery. Analysis was performed after anonymisation of data. The study was approved by the Ethics Committee of the University of Tübingen (341/2017BO2).
STATISTICAL ANALYSIS
Data are given as means±standard deviation. For the comparison of outcome, chi-squared or Fisher’s exact tests were used as appropriate. For all analyses, a two-tailed p-value <0.05 was considered statistically significant. All statistical tests were performed with SPSS Statistics software, Version 24.0 (IBM Corp., Armonk, NY, USA).
Results
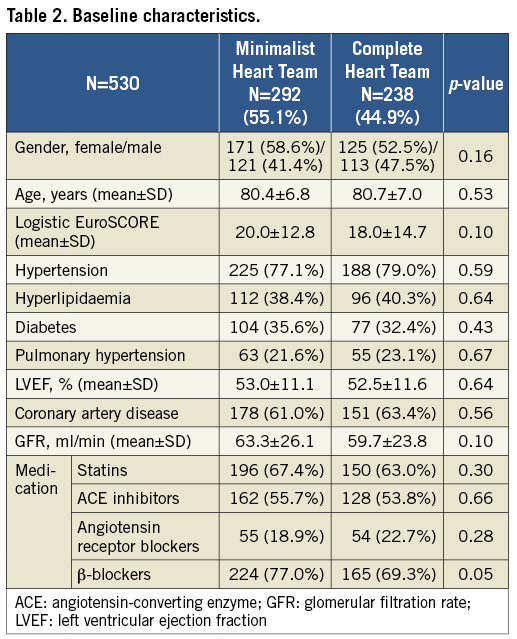
Two hundred and ninety-two patients were treated by the minimalist Heart Team, whereas, from August 2015, 238 patients were treated by the complete Heart Team. Baseline characteristics are shown in Table 2. The groups were comparable according to baseline characteristics and were in the high perioperative risk category (logistic EuroSCORE of 20 and 18 for the minimalist and complete Heart Team, respectively).
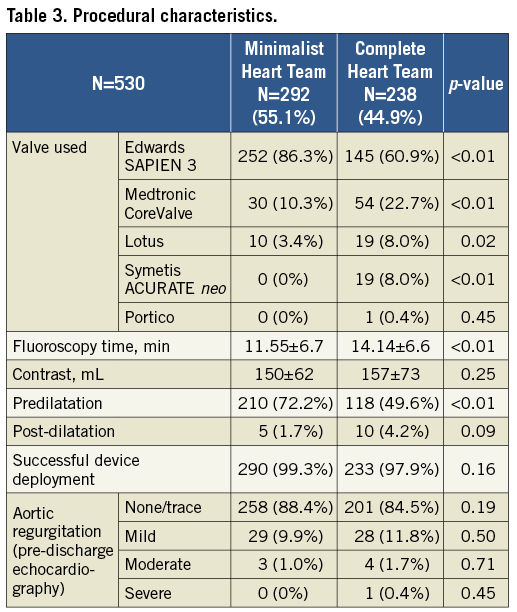
Prostheses were successfully implanted in 290 (99.3%) and 233 (97.9%) patients in the minimalist and complete Heart Team setting, respectively (p=0.16). Procedural characteristics are shown in Table 3. Edwards SAPIEN 3 valves (Edwards Lifesciences, Irvine, CA, USA) were mostly used, whereas there was significantly more frequent use of CoreValve® (Medtronic, Minneapolis, MN, USA), Lotus™ (Boston Scientific, Marlborough, MN, USA), ACURATE neo™ (Symetis SA, Ecublens, Switzerland) and Portico™ (St. Jude Medical, St. Paul, MN, USA) prostheses in the complete Heart Team group. There were no significant differences in contrast use; however, the fluoroscopy time was significantly longer in the complete Heart Team group. Predilatation was more frequently used in the minimalist Heart Team group. Significant aortic regurgitation after the procedure was very rare, showing no significant differences between the groups (Table 3).
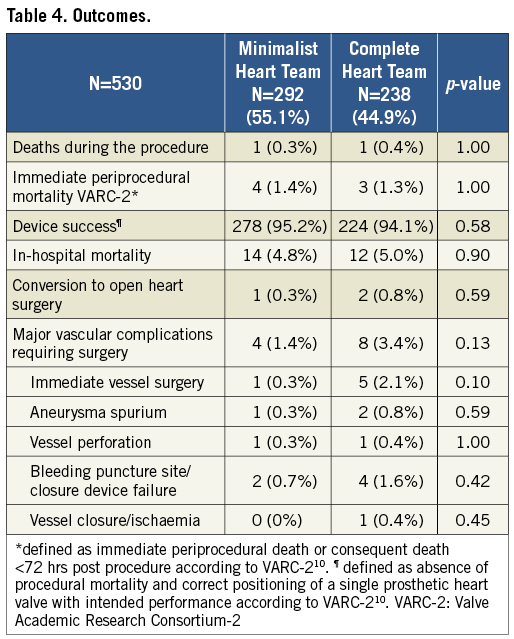
There were two deaths during the procedure, one in the minimalist and one in the complete Heart Team setting (0.3% vs. 0.4%, respectively, p=1.0) (Table 4). One patient suffered perforation of the descending aorta due to kinking during valve implantation and died of haemorrhagic shock despite an attempt at interventional treatment with balloon occlusion and stent graft implantation (minimalist Heart Team setting). The second patient died due to cardiogenic shock caused by extensively impaired left ventricular function and aortic regurgitation during prosthesis implantation. Because of kinking and venous obstruction, it was impossible to implant ECLS. The patient was not converted to surgery following an interdisciplinary decision (complete Heart Team setting). Furthermore, five patients died <72 hrs after the procedure (asystole, pericardial effusion, perforation of iliac artery, puncture site bleeding, thromboembolic myocardial infarction), resulting in a VARC-2 defined immediate periprocedural mortality of 1.4% and 1.3% for the minimalist and complete Heart Team, respectively (p=1.0). Consequently, the VARC-2 defined device success was 95.2% and 94.1% in the minimalist and complete Heart Team setting, respectively (p=0.58). There were three conversions to open heart surgery during the TAVR procedure, one in the minimalist and two in the complete Heart Team setting (0.3% vs. 0.8%, p=0.59). One patient suffered perforation of the iliac artery and was converted to surgery with vessel reconstruction and surgical aortic valve implantation. Despite successful surgery, he died shortly after due to haemorrhagic shock (complete Heart Team setting). Two patients had ventricle perforation during successful valve implantation (one each in the minimalist and complete Heart Team setting). Both patients with ventricle perforations underwent pericardiocentesis immediately after diagnosis of relevant pericardial effusion but had to be converted to open heart surgery due to persistent bleeding. Despite successful ventricle suture, one patient died 11 days following the surgery after a prolonged ICU course due to comorbidities (minimalist Heart Team). We did not observe any annulus rupture or direct coronary obstruction in either cohort. There were no significant differences regarding overall in-hospital mortality (4.8% vs. 5.0% in the minimalist vs. the complete Heart Team, respectively, p=0.9) or major vascular complications requiring surgery in either group (1.4% vs. 3.4%, respectively, p=0.13).
Discussion
An increasing number of patients with aortic stenosis are treated with TAVR. In recent years, with increasing experience and technical device enhancements, TAVR is still more often performed using a minimalist approach. Performing the procedure under local anaesthesia is non-inferior to the standard of using general anaesthesia and furthermore can be safer. In a recent meta-analysis, TAVR under local anaesthesia was associated with lower 30-day mortality, shorter procedural and fluoroscopy time, shorter ICU and hospital stay and reduced inotropic support6. Furthermore, more patients at intermediate risk are now treated with TAVR, consequently reducing the risk of serious adverse events. For instance, 30-day mortality ranges from 5% in PARTNER11 to 4% in PARTNER 212 in patients at intermediate risk. Still more patients are treated on an overnight basis13. With a growing number of centres performing TAVR under local anaesthesia, attendance of the complete Heart Team including anaesthesiologists and heart surgeons was omitted in some clinics14. There are several advantages of this setting including cost reduction and simplifying the logistics of the TAVR procedure. On the other hand, there are safety concerns regarding the minimalist design. Even though rare, severe complications necessitating immediate surgical conversion such as perforation of major vessels, ventricle perforation or annulus rupture still occur. Delaying the surgery and inappropriate intensive care management could have fatal consequences with higher perioperative mortality or worse clinical outcome. However, the safety and efficacy of this setting has not been studied extensively. In some smaller retrospective analyses, performing TAVR without the attendance of an anaesthesiologist did not change in-hospital outcome14-16.
In our larger analysis with 530 patients, we could not find any differences in perioperative complications and serious adverse events in patients treated by TAVR in the setting of a minimalist Heart Team omitting the presence of a cardiac surgeon and an anaesthesiologist. However, to minimise this risk of the procedure in the minimalist Heart Team, we performed all TAVR in hybrid operating rooms allowing for rapid conversion to open heart surgery without transporting the patient. Furthermore, the surgical team was available within the clinic rather than off site, with short arrival times in case of severe complication with necessary surgical management. Thanks to this organisation, rapid conversion to open heart surgery could also be obtained without the direct attendance of the thoracic surgeon or anaesthesiologist. Serious periprocedural complications were very low, with periprocedural mortality of approximately 1% in high-risk patients in both groups. Only three patients were immediately converted to open heart surgery.
Limitations
There are several limitations of our study. Due to its retrospective design, there were some significant differences in periprocedural characteristics, namely the use of more prosthesis types and less predilatation in the complete Heart Team setting. This reflects the increasing experience of operators and market development with the availability of more prostheses as well as emerging evidence supporting direct valve implantation17. However, due to the similarity of the implantation technique, this would probably not affect fatal peri-interventional complications. Severe complications were rare in our study, which limits its statistical power. However, to the best our knowledge, this is the largest study so far analysing different team settings during the TAVR procedure.
Conclusions
In conclusion, we could not find a difference in severe perioperative complications with the minimalist Heart Team approach. This study supports the strategy of reducing personal requests for TAVR under local anaesthesia, with the omission of the direct attendance of a surgical and anaesthesiological team during the procedure. However, due to possible severe complications necessitating surgical intervention, TAVR should be performed in experienced centres with surgical back-up. Prospective randomised studies concerning perioperative settings are warranted.
| Impact on daily practice In our retrospective single-centre study, we could not find a difference in severe perioperative complications using a minimalist Heart Team approach in TAVR under local anaesthesia. This study supports the strategy of reducing personal requests for TAVR, with the omission of the direct attendance of a surgical and anaesthesiological team during the procedure. |
Acknowledgements
We acknowledge the help of Jane Gollub with the preparation of the manuscript.
Funding
This study was in part funded by the DFG (German Research Foundation) – Project 374031971 – TRR 240.
Conflict of interest statement
M. Droppa has received travel honoraria from Medtronic. M. Gawaz has received speaker’s fees from Medtronic and AstraZeneca. T. Geisler has received restricted grants from Edwards and travel honoraria from Medtronic. The other authors have no conflicts of interest to declare.

