Abstract
As transcatheter aortic valve implantation (TAVI) becomes a standardised procedure with reproducible and excellent periprocedural, early and medium-term outcomes, it is opportune to question whether the time has come to simplify the TAVI procedure. In some centres, a minimalist approach to TAVI is already the standard of care. In this perspective, we share our experience and opinions on how and when we can simplify the TAVI procedure.
Introduction
Transcatheter aortic valve implantation (TAVI) has completely changed and revolutionised how we treat valvular heart disease. TAVI was conceptualised in order to simplify aortic valve replacement from requiring open heart surgery to a less invasive transcatheter approach which could be performed in the cathlab. Currently, over 80,000 TAVI procedures have been performed worldwide. The procedure has become fairly standardised and reproducible, procedural success rates are high and clinical outcomes are comparable to the gold standard of surgical aortic valve replacement1-3. However, practices regarding how the procedure is performed vary widely with large differences on either side of the Atlantic Ocean and with TAVI being performed in many centres with resource utilisation and costs which far outweigh surgical aortic valve replacement. Thus, 12 years after Alain Cribier performed the first human implant under local anaesthetic and sedation4 and pioneered a minimalistic approach to TAVI5, it is timely to question whether we should simplify the TAVI procedure.
THE MOST IMPORTANT GOAL IS SUCCESS WITH SIMPLICITY: DO NOT FORGET THAT SUCCESS COMES FIRST!
For most of our patients TAVI is a “once in a lifetime procedure”. This statement means that the immediate and the long-term results are the most important aspects, and every effort needs to be made to achieve an optimal implantation. As in many other procedures, the actual execution is an intermediate step preceded by planning and followed by post-procedural care. For this reason, we would like to dissect the TAVI procedure into three phases: pre-procedural planning, valve implantation and post-procedural care. We would also like to split our recommendations into two categories (Table 1). Category 1 incorporates suggestions which can be implemented in most patients undergoing TAVI, while category 2 lists suggestions to be put into place only in selected subgroups of patients. We would like to point out that most of the suggestions highlighted in this review bear a level of evidence “C” and that the entire field is in evolution. Furthermore, many of these recommendations may be more appropriate when the TAVI team has gained a certain level of experience and overcome the initial learning curve.
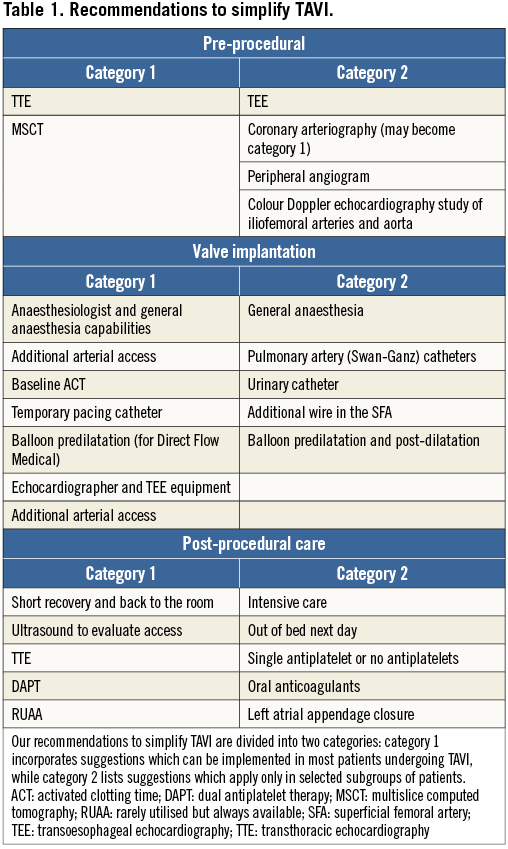
Pre-procedural planning
The first step lies in the correct diagnosis of aortic stenosis (AS). We would like to assume that we are dealing with calcific senile AS and we will not take into consideration paediatric cases and/or rheumatic AS. We are also assuming that the patient we are evaluating is an appropriate candidate and qualifies to undergo TAVI using current Heart Team clinical criteria.
TRANSTHORACIC ECHOCARDIOGRAPHY
Transthoracic echocardiography (TTE) and a Doppler study are the initial steps and these two tests cannot be skipped. Besides giving us the diagnosis of AS, these tests will give us important additional information. Some important data which TTE provides include the presence of a bicuspid aortic valve, coexistent aortic regurgitation, left ventricular dysfunction, or other valvular heart disease. However, computed tomography (CT) is better for detecting bicuspid AS, especially functional or pseudo-bicuspid valves, i.e., three sinuses and leaflets but with a fused commissure. We are fully aware that TAVI with the current transcatheter heart valves in bicuspid aortic valves is feasible but the procedure is more challenging and the results require improvement. Thus, having this information from the very beginning may influence the initial decision to proceed with TAVI. In some situations, such as aortic stenosis with low flow and low gradient, the TTE may need to be performed with dobutamine stimulation (TTE is a category 1 test).
TRANSOESOPHAGEAL ECHOCARDIOGRAPHY
A transoesophageal echocardiogram (TEE) is a test which will be performed in most patients. TEE may not have the universal weight of TTE; nevertheless, the additional information obtained with a TEE may be important and for this reason we will give TEE a position among the tests in category 1. Aware of this statement we would like to add that, in some situations and according to local practice, routine performance of a TEE exam is not universally mandated when the TTE evaluation is very clear and a multislice CT (MSCT) has given a detailed description of the anatomy.
MSCT
Nowadays, MSCT is a test performed as a matter of routine in most patients undergoing TAVI and is a category 1 test. While we strongly endorse the performance of MSCT and its importance in pre-procedural planning, sizing and selection of prosthesis, there are nevertheless situations where the above statement needs a revision. In patients with severe kidney compromise such as eGFR <30 cc/min there is an absolute need to limit contrast injection. In addition, every effort should be made to hydrate the patient fully, and to utilise appropriate renal protection protocols according to the practice of each specific institution. Furthermore, contrast load can be decreased by calculating the optimal angiographic plane with the MSCT and by diluting contrast media (40-50%), which is able to guarantee an acceptable visualisation in large anatomies. In these situations, MSCT can be focused on the evaluation of the thoracic aorta and the annulus with a reduced amount of contrast. A difficult decision in these patients is how to optimise the evaluation of the coronary tree. A selective angiogram with 20 cc of contrast is the most practical solution. The most important pieces of information to be obtained by MSCT are annular size, depth and width of coronary sinuses, distance from annulus to coronary ostia, diameters of sinotubular junction, as well as pattern and severity of valvular and subvalvular calcification6,7. MSCT evaluation can also give the ideal angulation (right vs. left and cranial vs. caudal) to align the three coronary sinuses optimally3. Information regarding the abdominal aorta, iliac and femoral vessels is important but, if saving contrast is an issue, a peripheral Doppler ultrasound exam can frequently give useful data.
SELECTIVE CORONARY ANGIOGRAPHY
Selective coronary angiography is usually performed in most patients undergoing TAVI. There are situations where the reading of MSCT is so clear as to exclude the presence of coronary artery disease and therefore to justify the omission of a coronary angiogram. For this reason we like to put coronary angiography in category 1 with the possibility of becoming category 2 in selected cases. Arteriography of the iliofemoral vessels is occasionally performed when the MSCT gives femoral diameters considered not fully suitable for a femoral approach. It is not rare to demonstrate with angiography that the evaluation obtained with MSCT was too pessimistic and that the procedure can actually be performed transfemorally.
PERIPHERAL ANGIOGRAPHY
A peripheral angiogram of the iliofemoral vessels can sometimes be necessary when MSCT or an echo Doppler study gives ambiguous results (category 2). If a coronary angiogram is performed prior to the procedure, an iliofemoral angiogram may give the information usually acquired with MSCT.
Valve implantation
The first question we would like to address regarding valve implantation and the procedure is: general anaesthesia versus conscious sedation. Anaesthetic management during TAVI has been the subject of considerable debate. Without question, a prior evaluation by an anaesthesiologist and his/her physical presence throughout the entire procedure are a must. This fact does not mean that general anaesthesia should be the standard. We would like to suggest general anaesthesia in selected patients who may have problems in tolerating the catheterisation laboratory environment or in patients with compromised haemodynamic status where full control may be preferable. Advantages of conscious sedation and local anaesthesia include more stable haemodynamics, less need for inotropic and/or vasopressor support, lack of endotracheal intubation and ventilation, awake patients who can alert the operator to an impending complication or the cause of a complication, shorter procedural duration, no requirement for ICU, and possibly a shorter hospital stay5,8. Usually, it is wise to have a discussion between the main operator and the anaesthesiologist regarding the decision to utilise general anaesthesia versus conscious sedation. For these reasons we give general anaesthesia a category 2 position. Interestingly, some centres have already moved away from this in that the anaesthesiologist is not physically present in the catheterisation laboratory during the procedure5.
An additional arterial access site is routine and can be radial or femoral. This need is a must with no exceptions because a pigtail in the right or non-coronary sinuses is needed to guide appropriate valve implantation. Even when continuous TEE monitoring is available, the presence of a catheter in the coronary sinus is a very useful landmark (category 1).
We usually utilise the additional arterial access to position a 0.018” wire in the superficial femoral artery on the side that will be utilised for the large introducer needed to deliver the valve. This extra wire will be needed to advance a “sealing” balloon to facilitate haemostasis, seal a pseudoaneurysm in the event of vascular device closure failure, or deliver a stent if iliac or femoral perforation occurs. The recent downsizing of sheaths to 14 Fr for some new-generation valves has given more confidence to the management of the arterial puncture, and this protection wire is now not routinely utilised (category 2). A simplification of the crossover technique for vascular access management is the placement of a 4 Fr sheath below the therapeutic puncture site. This can be used to perform final angiography, can be rapidly upsized if covered stent implantation is required and simplifies management of access-site complications, particularly if the operator has limited peripheral interventional experience.
We always perform a baseline activated clotting time (ACT) before heparin has been administered. It is not rare to detect an increased baseline ACT value. Heparin is our anticoagulant of choice and it is always administered immediately after obtaining the therapeutic access. The usual dose of heparin is 60 units/kg. We administer a lower dose of heparin when a longer baseline ACT has been detected9.
A temporary pacemaker catheter is always positioned in the right ventricle (category 1). We tend to utilise the jugular approach, especially when self-expanding valves are implanted. This access site allows patient mobilisation even when the temporary lead stays in place for a few days. We prefer to utilise balloon-tipped pacemaker catheters leaving the balloon inflated to minimise the risk of ventricular perforation10. However, if a non-balloon-tipped or helical screw temporary lead is used, we strongly recommend that a steep left anterior oblique view is used to ensure that the tip of the catheter is pointing towards the right ventricular septum and not towards the right ventricular free wall11.
Aortic valve balloon predilatation is presently mandatory in all cases only when implanting a Direct Flow Medical® valve (Direct Flow Medical, Santa Rosa, CA, USA) (category 1). When utilising balloon-expandable valves or self-expanding valves, the operator will decide whether to perform balloon predilatation, though at present this is rarely utilised (category 2). The need to perform balloon post-dilatation depends on the degree of aortic regurgitation following valve implantation and upon the cause of the regurgitation. After accessing the left ventricle, close attention to optimal positioning (right anterior oblique projection) and wire stability are critical to minimise the risk of perforation.
An experienced echocardiographer is always available during each TAVI procedure. Echocardiography is needed to evaluate the possible cause of a complication and to establish the degree of residual aortic regurgitation (category 1). TTE is the default exam, while TEE is performed only when the TTE window is not adequate or a specific issue needs to be evaluated and the echo operator considers TEE necessary. TEE can be performed for a short time interval and only light sedation is needed. Nevertheless, TEE capabilities should be available during all TAVI procedures because the precise and prompt evaluation of some complications or aortic regurgitation can sometimes only be established with an immediate TEE.
Percutaneous vascular closure is now a very predictable procedure, which is simplified by the ProGlide system (Abbott Vascular, Santa Clara, CA, USA) which entails advancing the device with preset knots. In a few rare situations, the knots detach from the artery and even an additional ProGlide device fails to achieve acceptable arterial sealing. Before getting ready for covered stent implantation or for vascular surgery repair, the crossover balloon should be inflated at the bleeding site and, following one-minute balloon occlusion, the manoeuvres we will describe should be put into action. After one minute of balloon occlusion, a full protamine dose should be given to reverse heparin and, at this point, gradual balloon deflation to 0.5 atm over one to two minutes should be performed. With the balloon inflated to 0.5 atm, a distal contrast injection performed via the balloon central lumen (0.035” compatible occupied by a 0.018” wire) should confirm acceptable distal run-off. A further confirmation is given by a pressure curve recorded at the tip of the balloon inflated at very low pressure. The partially inflated balloon should be kept for at least 10 minutes. If this manoeuvre fails, covered stent implantation or vascular surgery consultation should be effected.
Pulmonary artery catheters and urinary catheters have already been abandoned in many centres.
During the procedure, close communication between the anaesthesiologist and the primary operator is essential, and judicious use of vasopressors should be made to avoid hypotension that may be difficult to revert.
Post-procedural care
Following valve implantation, the patient is monitored in a recovery room for about two hours and, if stable, the patient is then transferred back to his/her room, which should have electrocardiographic telemetric monitoring available for at least 48 hours (category 1). In selected cases, such as complex implantation procedures, complications during the procedure, procedures performed under general anaesthesia with endotracheal intubation or when dealing with haemodynamically unstable patients, we consider transferring the patient to the intensive care unit (category 2). Unless contraindicated, most patients are out of bed the following day (category 2) and an ultrasound study is performed to evaluate the access site (category 1). However, in patients with uncomplicated procedures, same day mobilisation and early discharge one to three days after the procedure is becoming the standard of care (category 1). A TTE is always performed prior to discharge (category 1), and all patients are given follow-up appointments to the valve clinic at one, six and 12 months (category 1). The following parameters should be taken into consideration to plan early discharge: patient factors such as EuroSCORE, STS score and degree of physical independence of the patient prior to the procedure; procedure-related aspects such as duration, complications, final result, specific experience with the device implanted; clinical conditions and laboratory parameters of the patient in the 24 hrs following the procedure; type and level of general support available to the patient following discharge12.
Regarding antiplatelet therapy, dual antiplatelet therapy is prescribed for three months, and then only aspirin is continued indefinitely (category 1). A single antiplatelet agent or sometimes no antiplatelets are given when the patient has severe thrombocytopaenia (less than 70,000) or sustained high-risk bleeding (category 2).
For patients with atrial fibrillation requiring oral anticoagulants (INR 2-2.5), a single antiplatelet (category 1) or no antiplatelet agents should be given (category 2). In selected patients with atrial fibrillation and with a high-risk profile for bleeding, closure of the left atrial appendage can be performed (category 2). We would like to reinforce that this field is in evolution and, with time, some recommendations will change.
Rarely utilised but always available (RUAA), all in category 1
– Cardiothoracic surgical team with operating room readily accessible
– Heart and lung cardiopulmonary bypass with percutaneous cannulas
– Vascular surgery team with operating room readily accessible
– Pericardiocentesis set
– All equipment necessary to perform emergency percutaneous coronary interventions
– Self-expandable peripheral covered stents
– Aortic occlusion balloons
TAVI ad hoc: “Prima Vista TAVI”
The idea of performing ad hoc TAVI immediately after a diagnostic angiography may sound crazy13, a notion to be aborted from the very beginning. Maybe we should stop writing at this point with the conclusion that this concept should not be explored because it is wrong. Nevertheless, no established punishment is usually set for expressing provocative or wrong ideas and this is the reason why we will expand our concept of “Prima Vista TAVI”.
The diagnosis of AS is first a clinical diagnosis obtained with history and physical examination. The confirmation can be easily obtained by crossing the aortic valve with a catheter. A left ventriculogram can also be performed to give additional data. An echocardiographer to perform TTE in the catheterisation laboratory could supply more anatomic details. A TEE can approximate the size of the aortic annulus. Coronary angiography and an aortogram are part of the standard work-up. Aware of the fact that we are undersizing the aortic annulus, the decision to implant an oversized self-expandable valve would not be completely wrong. Before inserting a large femoral sheath and before preparing the valve, an angiogram of the iliofemoral vessels could be performed. If borderline anatomy is found, the vascular access can be improved by predilating the femoral and iliac arteries with a 7 or 8 mm non-compliant balloon. At this point we are set to go. After having crossed the aortic valve and most of the time without the need to predilate, the self-expanding valve is deployed. The echo and or aortogram will dictate if post-dilatation is needed. We will utilise a balloon sized according to echo because we know it will be undersized and therefore safe. If we are careful with our injections and use diluted contrast when performing large vessel imaging, we can complete the entire procedure with less than 150 cc of dye. Access closure will be performed as usual and “voilà, TAVI is done!”
However, while it may be amusing and entertaining to speculate that TAVI may be performed as we perform ad hoc PCI today, we are not suggesting or recommending that this be attempted today with all the current devices and in unselected patients.
In addition, it is fundamental that, when such an approach is taken, a prior discussion should take place with the patient, the family and with the components of the Heart Team.
Cost-effectiveness of TAVI
While TAVI may reach cost-effectiveness in inoperable patients provided life expectancy reaches at least 2.5 years, the issue is less well defined in high and medium-risk cohorts14,15. A recent report suggests that utilising a minimally invasive approach with only conscious sedation TAVI may save costs compared to a traditional approach to TAVI16,17. The current main barriers to improving cost-effectiveness of TAVI versus standard surgical valve replacement are: the cost of the transcatheter aortic valves and the high-risk profile of some patients treated with this modality requiring a prolonged and more complex hospital stay. Factors which may favour cost-effectiveness of TAVI are the fact that most of the patients can be treated with a short hospital stay with reduced or no need for intensive care admission and minimal or no rehabilitation18.
Conclusions
Simplifying the TAVI procedure is not speculative but many of the areas discussed are already standard practice in many centres. In experienced centres, this simplified or minimalist approach to TAVI is as safe and effective as the more standard traditionalist approach. While some critics may view this approach as an effort to dismantle the Heart Team, we think that it may actually result in a more efficient utilisation of resources without excluding the input of all the members of the team. This minimalist approach may also result in lower procedure-related costs and shorter hospital stays. Another critique may be the impact of oversimplification when treating moderate-risk patients where complications are unacceptable. However, in our experience this approach to TAVI is associated with similar outcomes to surgery, even in intermediate-risk patients19. Finally, the introduction of dedicated guidewires, easily inflated/deflated non-compliant aortic valvuloplasty balloons, new vascular closure devices, a continued reduction of valve profiles, and devices which facilitate accurate positioning while at the same time eliminating PVL will continue to simplify the TAVI procedure.
Conflict of interest statement
I. Michev is a consultant for Medtronic CoreValve. A. Latib is a consultant for Medtronic and Direct Flow Medical. A. Colombo is a minor shareholder of Direct Flow Medical.

