Abstract
Background: Compared with everolimus-eluting metallic stents, the Absorb bioresorbable scaffold (BRS) results in increased rates of myocardial infarction (MI) and scaffold thrombosis (ST) during its three-year bioresorption phase. It is unknown whether prolonged dual antiplatelet therapy (DAPT) duration might decrease the risk of ischaemic events.
Aims: We sought to evaluate the impact of DAPT duration on ischaemic and bleeding outcomes following BRS implantation.
Methods: We conducted an individual patient data pooled analysis from four ABSORB randomised trials and one prospective ABSORB registry. Study endpoints were MI, ST, bleeding, and death up to three-year follow-up. Propensity score-adjusted Cox regression analysis was used to account for baseline differences related to DAPT duration.
Results: The five ABSORB studies included 2,973 patients. DAPT use was 91.7%, 53.2%, and 48.0% at 1, 2, and 3 years, respectively. DAPT use within the first year after BRS implantation was associated with markedly lower risks of MI (adjusted hazard ratio [aHR] 0.17, 95% CI: 0.10-0.32; p<0.0001) and ST (aHR 0.08, 95% CI: 0.03-0.19; p<0.0001). Conversely, DAPT use between 1 and 3 years did not significantly affect the risk of MI (aHR 1.04, 95% CI: 0.70-1.55; p=0.84) or ST (aHR 0.86, 95% CI: 0.42-1.75; p=0.67). DAPT did not have major effects upon bleeding or death in either period.
Conclusions: DAPT use during the first year after BRS implantation was strongly associated with lower risks of ST and MI. However, a benefit of ongoing DAPT use between 1 and 3 years after BRS implantation was not apparent.
Introduction
First-generation Absorb™ (Abbott Laboratories, Abbott Park, IL, USA) poly-L-lactic acid (PLLA)-based everolimus-eluting bioresorbable scaffolds (BRS) failed to show non-inferiority compared with cobalt-chromium fluoropolymer-based everolimus-eluting stents (EES) due to higher rates of scaffold thrombosis (ST), target vessel-related myocardial infarction (MI), and target lesion revascularisation1,2. However, a recently published, individual patient data (IPD) pooled analysis from four ABSORB randomised trials demonstrated that the risk period was confined to the first three years after BRS implantation, the approximate time of PLLA bioresorption. In particular, the incidence of ST with BRS was negligible during follow-up between 3 and 5 years (only one case of ST among 2,161 BRS-treated patients [<0.1%] in this two-year period, compared with a 2.4% ST rate between the time of implant and three-year follow-up)2. These data indicate that the period of excess risk for the first-generation Absorb BVS ends at approximately three years.
The use of prolonged dual antiplatelet therapy (DAPT) with metallic drug-eluting stents (DES) has been shown to reduce MI and stent thrombosis rates, at the cost of increased bleeding3. Prolonging DAPT after BRS implantation during the three-year risk period may thus mitigate their higher propensity for ischaemic events (ST and MI) during the bioresorption phase. However, the causes of very late ST and MI after BRS are multifactorial, and include novel mechanisms such as intraluminal scaffold dismantling4; whether prolonged DAPT is useful to prevent these BRS-related events is unknown.
We therefore sought to evaluate the impact of DAPT duration on ischaemic and bleeding events following BRS implantation up to three-year and five-year follow-up from a large individual patient data pooled analysis of four randomised controlled trials and one prospective registry.
Methods
PATIENT POPULATION AND INCLUDED STUDIES
We pooled IPD from five prospective studies into a common database, including four randomised controlled trials comparing BRS and EES (ABSORB II, NCT01425281; ABSORB Japan, NCT01844284; ABSORB China, NCT01923740; and ABSORB III, NCT01751906) and one single-arm BRS prospective registry (ABSORB EXTEND, NCT01023789). The principal analysis focused on three-year outcomes, the period of active scaffold bioresorption. A secondary exploratory analysis of five-year outcomes is also provided. This observational analysis followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (Supplementary Table 1). The main characteristics of these five studies are summarised in Supplementary Table 2. In all five studies, aspirin use was required indefinitely; use of a platelet receptor P2Y12 inhibitor was required for the first year after BRS implant, after which its administration was optional according to investigator discretion. In all of the studies the patients were queried for their continuous (i.e., daily) DAPT usage during follow-up.
ENDPOINTS AND DEFINITIONS
For the present study we were interested in evaluating the impact of DAPT duration during the high-risk period of the first three years following BRS implantation. We further pre-specified examining outcomes between 0 and 1 year after device implantation (the typical high-risk period for metallic DES, after which event rates are low), and between 1 and 3 years after device implantation, when the greatest hazards for BRS relative to DES were noted2. The outcome measures of interest for this study were MI (target vessel-related and non-target vessel-related), ST, bleeding, and all-cause death. Procedural and non-procedural MI were defined using the ABSORB III criteria1. ST was defined according to the Academic Research Consortium definite or probable criteria5. Bleeding was defined by the GUSTO classification of “life-threatening or severe” (either intracranial haemorrhage or bleeding that causes haemodynamic compromise and requires intervention) or “moderate” (bleeding that requires blood transfusion but does not result in haemodynamic compromise)6. All ischaemic events were adjudicated by an independent clinical events committee after review of original source documents. Bleeding events were site-reported and monitored but not adjudicated.
STATISTICAL ANALYSIS
Baseline characteristics are summarised using means and standard deviations for continuous variables, and as numbers and percentages for categorical variables. The principal analyses were performed from the time of BRS implantation up to three years (0-3 years), and between 0-1 year and 1-3 years separately. A secondary analysis was performed considering 3-5 year and 0-5 year events. Analyses were stratified according to DAPT usage. Event rates were estimated with the Kaplan-Meier method, and compared with the log-rank test.
A propensity score-adjusted analysis was used to account for differences in 11 patients and lesion variables that might have affected the decision to use long-term DAPT. The propensity score accounted for age, sex, diabetes, recent smoker (<1 month), acute coronary syndrome (versus stable coronary artery disease), total lesion length, smallest baseline reference vessel diameter, treatment of any calcified lesion, any bifurcation lesion, any lesion in the left main or left anterior descending coronary arteries, and the number of treated lesions. Daily DAPT use was entered into a propensity score-adjusted and study-level adjusted Cox multivariable model as a time-adjusted covariate, and the effect of permanent DAPT discontinuation (≥2 days, and until last follow-up or time of event) was assessed. The results of this analysis are presented as hazard ratios (HR) and 95% confidence intervals (CI). To examine the change in hazards during the three-year follow-up period further, a flexible parametric propensity score-adjusted survival model was used to estimate the HR and 95% CI for the study outcomes over time. An interaction term between DAPT status and a natural spline of the log of time with two degrees of freedom was included in the model. A p-value <0.05 was considered significant. All statistical analyses were performed with SAS, version 9.4 (SAS Institute, Cary, NC, USA).
Results
PATIENTS AND PROCEDURES
The five ABSORB studies included 2,973 patients (3,149 lesions) treated with Absorb BRS at 357 centres in America, Europe, Asia, and Oceania. Three-year follow-up data were available for 2,851/2,973 patients (95.9%). The baseline characteristics of the patients enrolled from each study have been reported previously2. Clinical, angiographic, and procedural characteristics across the five studies are presented in Supplementary Table 3-Supplementary Table 5, respectively. Mean age was 62.4±10.8 years, 73.0% of patients were male, 29.2% had diabetes, 23.3% prior MI, 31.7% prior PCI, and 31.5% presented with an acute coronary syndrome. A single lesion was treated in 94.0% of patients, 60.5% had a type B2/C lesion treated, predilatation was performed in 99.8%, post-dilatation with a non-compliant balloon was performed in 61.6%, and intravascular imaging guidance was used in 21.6%. Device success was achieved in 98.7% of lesions.
DAPT USE DURING FOLLOW-UP
Figure 1 shows data on aspirin, P2Y12 inhibitor, and DAPT use at several time points during follow-up. Among P2Y12 inhibitors, clopidogrel was used in the majority of patients (82.7%), followed by prasugrel (11.1%) and ticagrelor (6.3%). DAPT was used in 91.7% of patients at 1 year, 53.2% at 2 years, and 48.0% at 3 years. Whereas aspirin was maintained in the vast majority of patients at three-year follow-up (95.3%), there was a sharp drop in P2Y12 inhibitor use between one year (93.6%) and subsequent time points, such that at three-year follow-up only 51.1% of patients were still on one of these agents. Longer-term follow-up showed further slight reductions in the use of antiplatelet agents (particularly P2Y12 inhibitors), so that at five-year follow-up 39.8% of patients were still on DAPT.
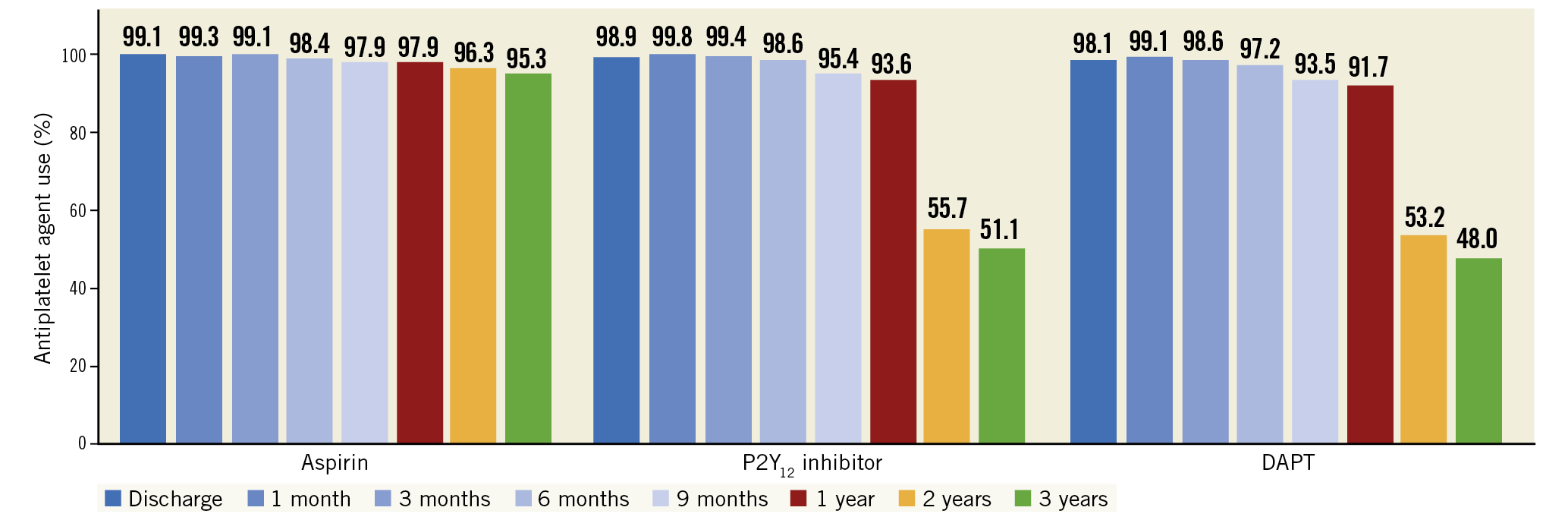
Figure 1. Aspirin, P2Y12 inhibitor, and dual antiplatelet therapy use at different time points during the three-year follow-up. Usage rates of each agent based on the total number of patients alive and on-study at that specific timepoint (i.e., end of interval), and thus the rates are different from those in Supplementary Table 7. DAPT: dual antiplatelet therapy
Supplementary Table 6 shows clinical, angiographic, and procedural characteristics according to DAPT status. Supplementary Table 7 presents data on the timing of DAPT discontinuation. While permanent DAPT discontinuation was uncommon (2.5%) within the first 6 months after implantation, its incidence rose to 16.5% between 6 and 12 months following the index PCI. DAPT was permanently interrupted in 44.5% of the study population between 1 and 2 years, in 51.4% of patients between 2 and 3 years, in 55.2% of patients between 3 and 4 years, and in 59.8% of patients between 4 and 5 years.
ADVERSE EVENTS ACCORDING TO DAPT USE DURING THREE-YEAR FOLLOW-UP
Table 1 presents the Kaplan-Meier estimates of pooled study outcomes. MI, ST, bleeding, and death occurred in 8.2%, 2.3%, 1.8%, and 2.9% of BRS-treated patients, respectively, during the three-year study period. In patients who developed MI or ST off DAPT within 1 year, the median duration from the time of last DAPT discontinuation to the event was 7.5 days, ranging from 0-334 days. In patients who developed MI or ST off DAPT between 1 and 3 years, the median duration from the time of last DAPT discontinuation to the event was median 451 days, ranging from 2-1,032 days.
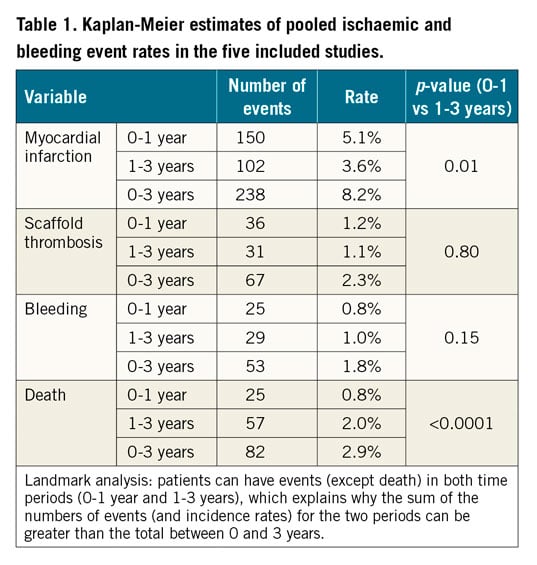
Table 2 shows the unadjusted relationships between permanent DAPT discontinuation and study outcomes. The Central illustration presents the propensity score-adjusted Cox regression analysis for the impact of daily DAPT status on outcomes. During the entire three-year period, DAPT use was associated with a non-significant effect on MI (HR 0.75, 95% CI: 0.50-1.10; p=0.14) but was strongly associated with reduced ST (HR 0.42, 95% CI: 0.21-0.84; p=0.01). However, these effects varied markedly between 0 and 1 year, and 1 and 3 years. During the first year after BRS implantation, use of DAPT was associated with a markedly reduced risk of MI (HR 0.17, 95% CI: 0.10-0.32; p<0.0001) and ST (HR 0.08, 95% CI: 0.03-0.19; p<0.0001). Conversely, DAPT use was not protective against MI (HR 1.04, 95% CI: 0.70-1.55; p=0.84) or ST (HR 0.86, 95% CI: 0.42-1.75; p=0.67) between 1 and 3 years. DAPT use had a weak and non-significant effect on bleeding throughout the study period. Finally, while DAPT use tended to confer protection from death within the first year after BRS implantation (HR 0.33, 95% CI: 0.09-1.14; p=0.08), this relationship tended to be reversed between 1 and 3 years (HR 1.70, 95% CI: 0.98-2.96; p=0.06), resulting in an overall neutral effect when considering the three-year follow-up as a whole (HR 1.31, 95% CI: 0.78-2.19; p=0.31).
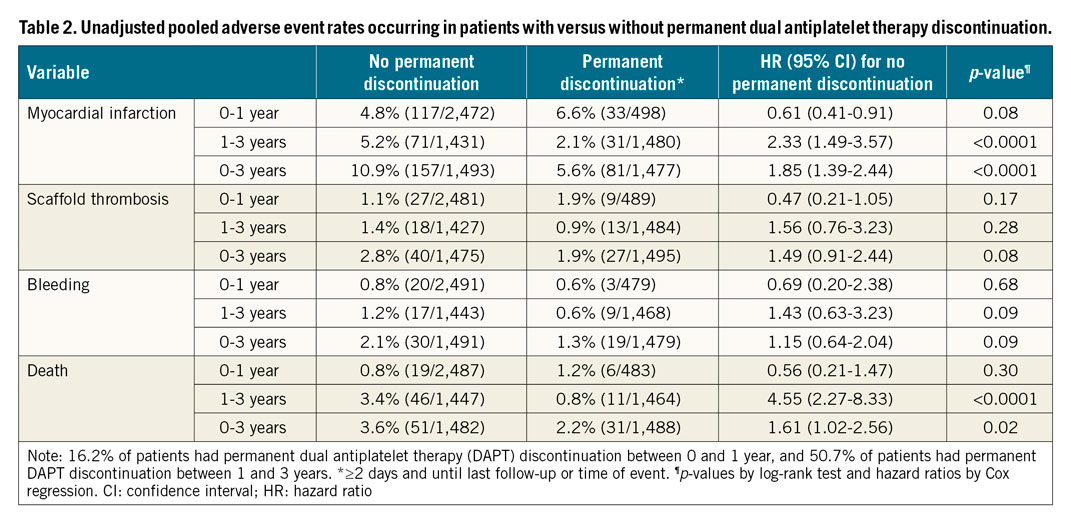
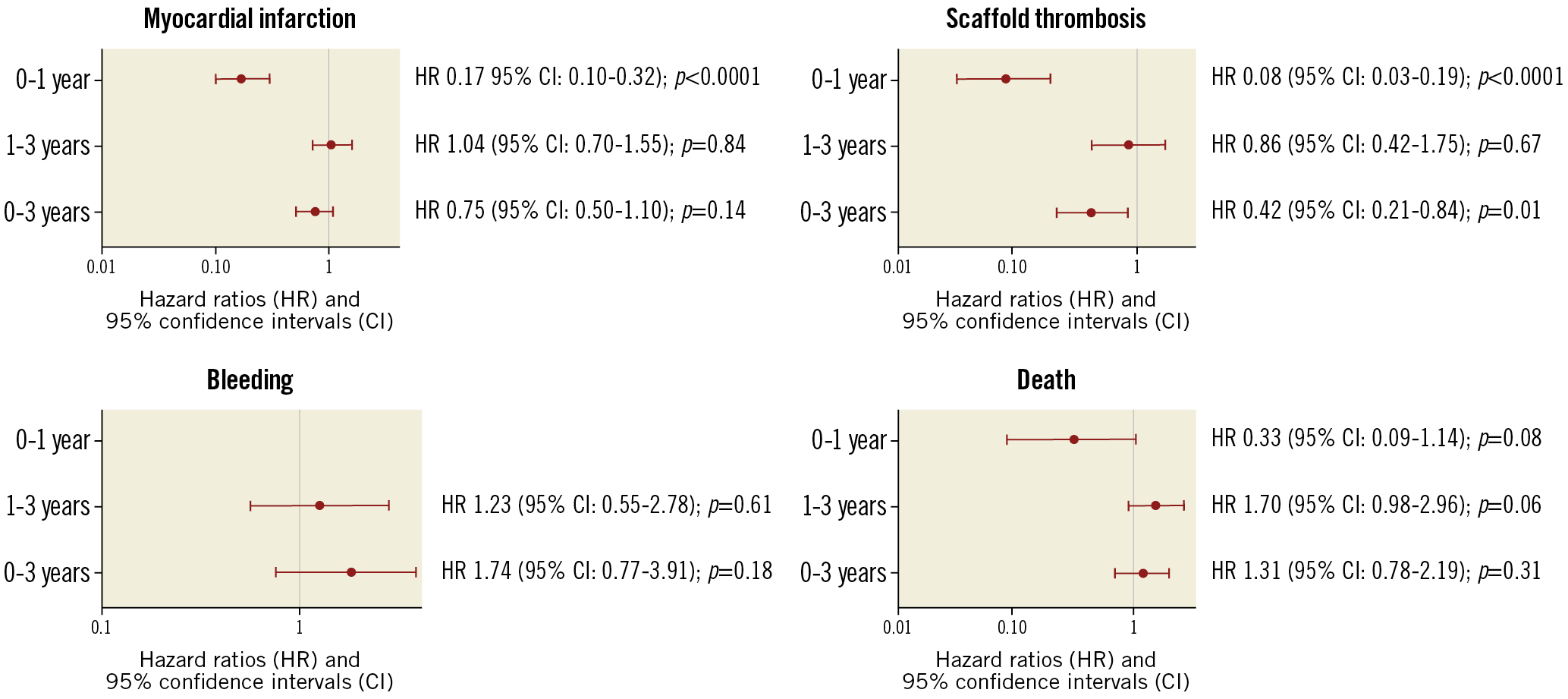
Central illustration. Propensity score-adjusted Cox regression analysis showing the impact of dual antiplatelet therapy on study outcomes. Analysis of the effect of permanent DAPT discontinuation on study outcomes. Estimates for the 0 to 1-year effect of DAPT on bleeding could not be calculated because of the low number of events.
By spline analysis (Figure 2), the adjusted HR for the association between DAPT use and MI was markedly low (<0.10) in the two months following BRS implantation, and later rose to stabilise at an HR of ~1.0 at 12 months, remaining constant until the end of the three-year follow-up. The upper bound of the 95% CI crossed 1.0 at approximately 4 months; after this time point the association between DAPT use and MI was non-significant. The unadjusted HR for ST was also very low (<0.10) until ~5 months after BRS implantation, and thereafter increased, stabilising at an HR of ~0.50 at 12 months up to the end of follow-up. The upper bound of the 95% CI approximated 1.0 at 12 months and beyond. Spline analysis indicated a small but non-significant risk of DAPT use for bleeding throughout the three-year study period. The findings for all-cause death also demonstrated non-significant associations throughout follow-up, although the HR tended to be below 1.0 for the first 6-8 months and above 1.0 thereafter.
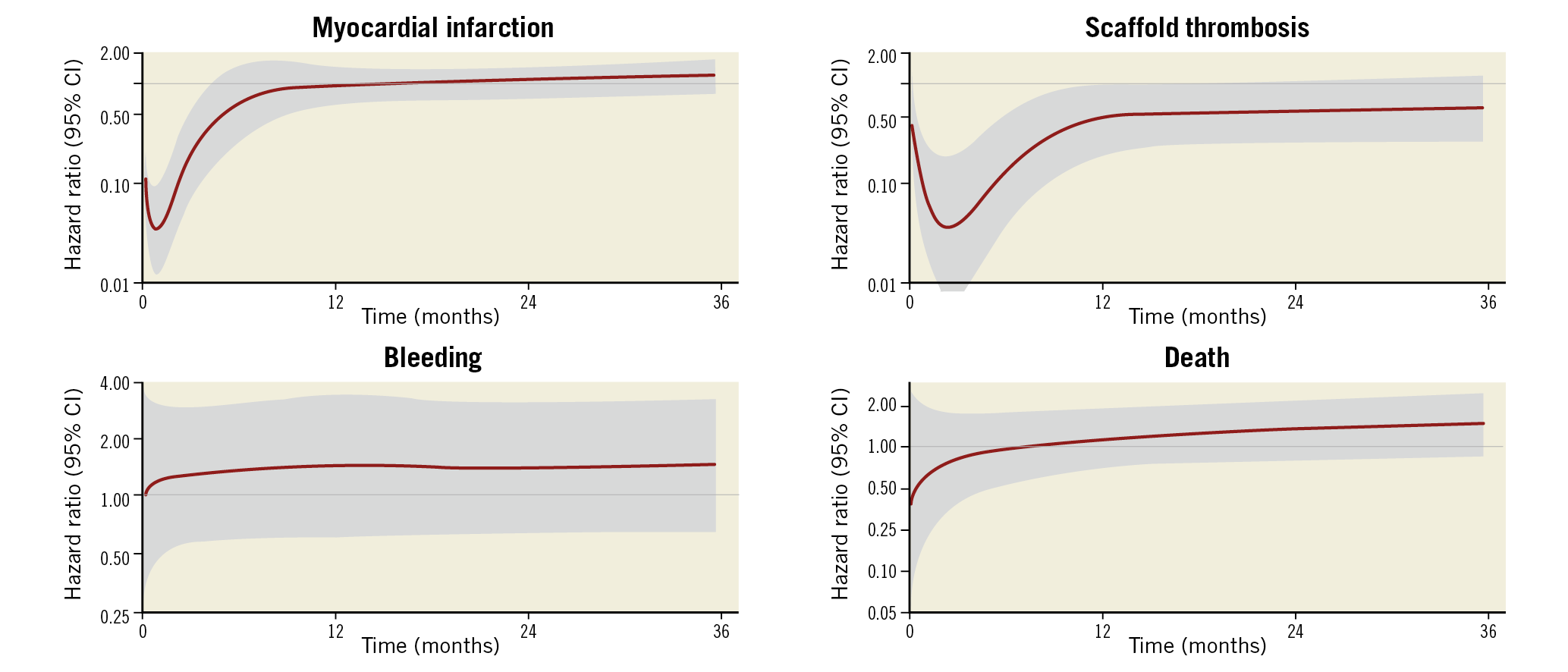
Figure 2. Spline analysis demonstrating the time-varying association of the hazard for study outcomes depending on dual antiplatelet therapy status during the three-year follow-up period. This analysis evaluates the effect of permanent dual antiplatelet therapy discontinuation on study outcomes. The solid red line represents the adjusted hazard ratio, while the grey shadow represents the 95% confidence interval. Analyses are covariate-adjusted for all outcomes, except for scaffold thrombosis which is unadjusted, as the adjusted spline model for scaffold thrombosis would not successfully converge due to the low number of events. CI: confidence interval
FIVE-YEAR OUTCOMES ANALYSIS
Our secondary analysis explored the impact of DAPT usage beyond three-year follow-up. Between 3 and 5 years, low event rates were observed for the outcomes of MI (2.0%), ST (0.1%), and bleeding (1.2%), while death was observed in 3.5% of patients overall. The 0-5 year estimates of the study endpoints were 9.9%, 2.5%, 3.1%, and 6.2%, respectively.
Supplementary Table 8 shows event rates as well as unadjusted and adjusted risks of the study endpoint in patients with versus without permanent DAPT discontinuation at 3-5 years and for the totality of follow-up (0-5 years). Event rates at 3-5 years in the permanent discontinuation group were very low (0-0.2%) for all endpoints, which led to unstable risk estimates. Considering the 0-5 year follow-up period in its entirety, there were no differences in the adjusted risks of MI, ST, bleeding or death between groups.
Supplementary Figure 1 shows the spline analysis including longer-term follow-up (up to five years). The findings extend those seen in the principal three-year analysis, with no significant adjusted differences between patients on versus off DAPT between 3 and 5 years. Of note, the spline model could not be fitted for the outcome of ST, as this event occurred in only 0.1% of patients in this period.
Discussion
The major findings from the present large-scale Absorb BRS IPD pooled analysis are: 1) while DAPT use was high (>90%) during the first year after BRS implantation, half or less of the study population remained on both aspirin and a P2Y12 inhibitor between 1 and 5 years; 2) permanent DAPT discontinuation during the first year after Absorb BRS implantation was associated with markedly higher adjusted risks of MI and ST; 3) conversely, DAPT discontinuation was not associated with increased ischaemic events between 1 and 3 years after BRS implantation (the period of active scaffold bioresorption), nor between 3 and 5 years (the period after complete scaffold bioresorption when event rates are low); and 4) DAPT use did not have effects on bleeding or all-cause death throughout the three-year study period.
Current guidelines recommend DAPT use for 6 months after metallic DES implantation in most patients with stable coronary artery disease, and for 12 months in most patients with acute coronary syndromes7. The rationale for these recommendations is that in prior studies the incremental bleeding risks from prolonging DAPT beyond these periods were greater than the additional ischaemic protection that was afforded. Whether these same recommendations are appropriate after BRS implantation is unknown and necessitates re-consideration of the relative risks of ischaemia versus bleeding over time with these novel devices. In this regard, the first-generation Absorb BRS compared with contemporary metallic DES has a larger footprint (strut thickness 157 µm) which may lead to greater platelet activation and delayed endothelialisation, incomplete support of the vessel wall during healing and scaffold resorption, and the development of scaffold discontinuities during the bulk resorption process with the potential for late intraluminal scaffold dismantling, all of which would be expected to prolong the ischaemic risk period4,8. In an IPD pooled analysis from the ABSORB II, ABSORB III, ABSORB China and ABSORB Japan randomised trials, treatment with Absorb BRS compared with EES was associated with higher rates of target lesion failure (TLF, 14.9% vs 11.6%; HR 1.26, 95% CI: 1.03-1.54) up to five-year follow-up2. However, the period of excess risk was limited to the first 3 years; between 3 and 5 years the rates of TLF were similar with BRS and EES. Moreover, device thrombosis was observed in 2.4% of BRS-treated patients versus 0.6% of EES-treated patients between 0 and 3 years (HR 3.86, 95% CI: 1.75-8.50), compared with 0.1% of BRS-treated patients versus 0.3% of EES-treated patients between 3 and 5 years (HR 0.44, 95% CI: 0.07-2.70). Significant time-dependent interactions were present for device use and these endpoints, indicating that the period of excess risk for the first-generation Absorb BVS ends when bioresorption is complete at ~3 years. Our principal analysis therefore assessed the impact of DAPT on adverse events after BRS for up to three years in the present study.
Prior studies examining the potential utility of prolonged DAPT after BRS have been limited to a modest number of patients and reported conflicting outcomes. Felix et al9 analysed 685 BRS-treated patients without early ST who took DAPT for >6 months. The ST incidence density was 0.26 versus 1.77 per 100 patient-years between 6 and 18 months after BRS implantation in patients on versus off DAPT, respectively, but rose to 6.57 per 100 patient-years within the first month after DAPT discontinuation. Of interest, all four cases of very late ST (>1 year after implantation) occurred in patients who had recently discontinued DAPT, and no case of very late ST occurred in patients who continued DAPT for >18 months. Similarly, in an early report from the ABSORB EXTEND registry10, 2 of the 4 ST cases were related to either premature DAPT termination or resistance to clopidogrel (all 4 ST events occurred during the first year after implantation). Likewise, among 810 Absorb BRS-treated patients in the Swedish SCAAR registry11, 6/11 patients who suffered ST within two years were non-adherent to DAPT, and all these events occurred within the first month following DAPT termination. All three very late ST events were observed in patients off DAPT, although there was no temporal relationship between DAPT discontinuation and ST in this period.
Compared to these prior reports, the present study was substantially larger, included patients across diverse geographic, ethnic, and practice-related spectra, and utilised multivariable analysis to isolate the potential role of DAPT in preventing adverse ischaemic events after Absorb BRS implantation. This analysis demonstrated that DAPT discontinuation during the first year after BRS implantation was strongly related to the occurrence of MI and ST. Conversely (and to our surprise), no clear beneficial effect of prolonged DAPT was apparent between 1 and 3 years after BRS implantation. Prolonged DAPT was associated with a non-significant risk of increased bleeding and a neutral effect on three-year mortality, although in this regard we cannot exclude a beneficial DAPT effect on survival within the first year and slight harm thereafter.
Several possible explanations may underlie the absence of benefit of continued DAPT use after one year in BRS-treated patients. First, in most cases endothelialisation of Absorb BVS is largely complete by 6 months12. After 12 months, the struts of adequately implanted (i.e., correctly sized, well-expanded and well-apposed) BRS are completely covered with neointima and thus less vulnerable to platelet adhesion. The very late risk of ischaemic events may thus be evidenced primarily in scaffolds which were not adequately implanted, and are thus prone to intraluminal scaffold dismantling4. In this regard, the greatest risk factor for TLF and ST after BRS implantation is in very small vessels, a risk which is confined to the first year8. Second, the individual studies comprising the pooled analysis enrolled mostly low-to-intermediate complexity lesions treated in our analysis: mean lesion length was only 13 mm, and the prevalence of overlapping scaffolds, bifurcation intervention, and moderate/severe calcification was low. Prolonged DAPT might have been associated with lower ischaemic events between 1 and 3 years, had a clinically higher risk or angiographically more complex patient population been studied. It may also be that the novel mechanisms related to very late ST and MI after BRS implantation are either platelet reactivity-independent, or conversely would require even more potent inhibition to prevent. As the vast majority of the study cohort was treated with clopidogrel, we are unable to assess this possibility. Finally, the lack of benefit of prolonged DAPT between 3 and 5 years following device implantation is probably explained by the low event rate after complete scaffold bioresorption, as previously reported3.
In our analysis, prolonged DAPT use had no significant relationship with bleeding. Bleeding, defined as GUSTO moderate or severe in the present study, was relatively infrequent in the current patient population from which many high bleeding risk patients were excluded. Although it is unlikely that bleeding events so defined would have been missed, we cannot exclude under-reporting of some episodes of less severe bleeding that did not require medical attention. The occurrence of such events would probably have demonstrated a relationship with prolonged DAPT, as seen in prior studies3.
Similarly, our adjusted analysis on the impact of prolonged DAPT on death was inconclusive. While DAPT usage tended to be associated with a lower risk of death in the first year (probably due to its salutary effects in preventing ST and MI), a non-significant negative association with prolonged DAPT use and survival was observed between 1 and 3 years. Prior studies have observed this phenomenon and correlated the late excess mortality risk directly to bleeding-related deaths13.
Limitations
First, as a non-randomised analysis, the results from the present study should be considered hypothesis-generating, as we cannot exclude the presence of unmeasured confounders. The occurrence of adverse ischaemic or bleeding events may also have dictated DAPT usage patterns, potentially affecting subsequent outcomes. Second, the specific reasons for DAPT discontinuation were not collected. Third, bleeding events from the component studies were not adjudicated by a clinical events committee. Fourth, the effect of DAPT use in the multivariable models was analysed according to its use the day before an adverse event. Sudden changes in DAPT usage were not accounted for, although few patients between 1 and 3 years had an MI or ST within 1 or 2 days after DAPT discontinuation. Fifth, DAPT utilisation was assessed from patient interview and, although these data were prospectively collected during regular follow-up visits, this is subject to imprecision in recall between visits14. Sixth, follow-up was not available in all patients, although the proportion of subjects lost to follow-up was low and unlikely to impact on overall study findings. Finally, the present results apply to the first-generation Absorb BVS, although the concepts outlined herein may be generalisable to other BRS prior to and after the time of their complete bioresorption.
Conclusions and clinical implications
In the present large-scale IPD analysis, DAPT use was strongly associated with a lower risk of MI and ST during the first year after Absorb BRS implantation. However, prolonged DAPT use had an uncertain risk/benefit profile between 1 and 3 years, and its continued use was not associated with a decrease in the risk of ischaemic outcomes in this period. The impact of prolonged DAPT use on bleeding and its net effect on mortality were neutral. No significant relationships between DAPT usage and adverse ischaemic or bleeding events were present between 3 and 5 years after device implantation, the time period after complete scaffold bioresorption when event rates are low. Randomised controlled trials are warranted to evaluate the risk/benefit profile of prolonged DAPT following implantation of novel BRS platforms, especially those with thinner struts or different bioresorption rates.
|
Impact on daily practice Dual antiplatelet therapy use during the first year after bioresorbable scaffold implantation was strongly associated with lower risks of scaffold thrombosis and myocardial infarction. However, a benefit of prolonged dual antiplatelet therapy use between 1 and 3 years after bioresorbable scaffold implantation was not apparent, as it did not significantly affect the risk of either outcome. Dual antiplatelet therapy did not have major effects upon bleeding or death in any period during follow-up. |
Funding
The ABSORB trials were funded by Abbott Vascular, Santa Clara, CA, USA. The present study was funded by a grant to the Cardiovascular Research Foundation from Abbott Vascular.
Conflict of interest statement
L. Azzalini has received honoraria from Teleflex, Abiomed, Philips, Asahi Intecc, Abbott Vascular, and Cardiovascular Systems, Inc. S. Ellis reports consulting fees/support for a meeting relating to the ABSORB study, and being a consultant for Medtronic and Biotronix. D.J. Kereiakes reports being a consultant for Boston Scientific, Sino Medical Sciences Technologies, Svelte Medical, and Elixir Medical. T. Kimura has received research support/honoraria from Abbott Vascular. R. Gao has received research support from Medtronic, MicroPort, and Lifetech Scientific. B. Chevalier reports receiving consultant fees/research grants from Abbott Vascular, being a consultant for Biotronik, Colibri, Medtronic, and Terumo, and being a shareholder of the Cardiovascular European Research Center (CERC). P.W. Serruys reports being a consultant for Biosensors, Sinomed, Balton Sp, Philips/Volcano, Xeltis, Novartis, Meril Life, and HeartFlow, and participation on the board of PROSPECT ABSORB. G. Stone reports payments to the institution from Abbott for biostatistics, clinical events committee and core laboratory work on the clinical trials and for support to attend meetings, speaker honoraria from Cook, being a consultant to Valfix, TherOx, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Miracor, Neovasc, V-Wave, Abiomed, Ancora, MAIA Pharmaceuticals, Vectorious, Reva, Occlutech, CoreFlow, Matrizyme, Shockwave, and Cardiomech, holding equity/options from Ancora, Qool Therapeutics, Cagent, Applied Therapeutics, the Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, Valfix, and Medfocus, and other financial or non-financial interests (Mount Sinai Hospital). Mount Sinai Hospital receives grants from Abbott for research. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

