
Abstract
Aims: The aim of this study was to investigate the impact of dual antiplatelet therapy (DAPT) termination on late and very late scaffold thrombosis (ScT) in patients treated with the Absorb bioresorbable vascular scaffold (BVS).
Methods and results: Data from the registries of three centres were pooled (808 patients). To investigate the effect of DAPT termination on ScT after a minimum of six months, we selected a subgroup (“DAPT study cohort” with 685 patients) with known DAPT status >6 months and excluded the use of oral anticoagulants and early ScT. In this cohort, definite/probable ScT incidence for the period on DAPT was compared to ScT incidence after DAPT termination. ScT incidence was 0.83 ScT/100 py with 95% confidence interval (CI): 0.34-1.98. After DAPT termination, the incidence was higher (1.77/100 py; 95% CI: 0.66-4.72), compared to the incidence on DAPT (0.26/100 py, 95% CI: 0.04-1.86; p=0.12) and increased within the month after DAPT termination (6.57/100 py, 95% CI: 2.12-20.38; p=0.01). No very late ScT occurred in patients who continued on DAPT for a minimum of 18 months.
Conclusions: The incidence of late and very late definite/probable ScT was acceptable. The incidence was low while on DAPT but potentially higher when DAPT was terminated before 18 months.
Abbreviations
ACS: acute coronary syndrome
BVS: bioresorbable vascular scaffold
CAD: coronary artery disease
DAPT: dual antiplatelet therapy
DES: drug-eluting stent
IVUS: intravascular ultrasound
MI: myocardial infarction
NOAC: new oral anticoagulant
NSTEMI: non-ST-elevation myocardial infarction
OAC: oral anticoagulant
OCT: optical coherence tomography
PCI: percutaneous coronary intervention
ScT: scaffold thrombosis
ST: stent thrombosis
STEMI: ST-elevation myocardial infarction
UA: unstable angina pectoris
VLScT: very late scaffold thrombosis
Introduction
Bioresorbable scaffolds are a new treatment option for coronary interventions with the aim of overcoming some of the limitations of metallic drug-eluting stents (DES), such as very late stent thromboses and reinterventions due to polymer reactions, strut fracture, neoatherosclerosis and inflammation.
The Absorb bioresorbable vascular scaffold (BVS) (Abbott Vascular, Santa Clara, CA, USA) has been the most intensively studied. Multiple meta-analyses have shown comparable one-year outcomes for target lesion failure (TLF) of the BVS versus the cobalt-chromium-based everolimus-eluting XIENCE stent (CoCr-EES) (Abbott Vascular) in selected patients. Numbers of scaffold thrombosis (ScT) and target vessel myocardial infarction (MI) tended to be higher in the BVS group1-3. In populations reflecting real-world patients4-8, ScT occurs more frequently. More recently, concerns have been expressed about the occurrence of very late (>1 year) scaffold thrombosis (VLScT)9,10. In randomised controlled trials (RCTs), VLScT rates up to two years were low in one (1.6%) but higher in another (2.0%) at three years11,12.
Dual antiplatelet therapy (DAPT) reduces the risk of local thrombotic events related to stent implantation, systemic thrombotic events, and cardiovascular mortality. In the current ESC and AHA/ACC guidelines, a minimum DAPT duration of six months after DES implantation is recommended, with prolonged treatment in patients with an increased risk of thrombotic events and low bleeding risks. For BVS, the optimal DAPT duration has not yet been clearly defined13,14. The early studies investigating BVS applied a minimum DAPT duration of six months. In the more recent RCTs, a minimum duration of 12 months was implemented11,15.
To summarise, data on long-term ScT outcomes after BVS implantation in real-world patients are lacking and information on optimal DAPT duration is missing. To fill this gap, we describe here the incidence of ScT and report our investigation into the impact of DAPT termination on late and very late ScT in regular clinical practice.
Methods
POPULATION
Patients were pooled from the registries of three Dutch centres where the Absorb BVS was used as part of daily clinical practice. The decision to treat a patient with BVS was made at the discretion of the interventional cardiologist.
The patients of the Erasmus Medical Center were drawn from two investigator-initiated, single-centre, single-arm registries (BVS Expand and BVS STEMI). Inclusion and exclusion criteria have been described elsewhere5,6. Patients included in the other two hospital registries were part of local all-comers registries initiated for the control of quality of standard care following introduction of a new CE-approved device.
Between September 2012 and April 2015, 808 patients treated with at least one BVS were included in this study (total cohort). To investigate specifically the association between DAPT and late events without the interference of oral anticoagulants, the DAPT cohort was selected by including patients with a known DAPT status and with a duration of at least six months, without the occurrence of early ScT and without usage of (new) oral anticoagulants ([N]OAC).
ETHICS
This is an observational study, performed based on international regulations, including the Declaration of Helsinki. Data were collected in an encrypted database with the approval of the local ethics committee. The Absorb BVS has received the CE mark and the BVS can be currently used routinely in Europe in different settings without a specific written informed consent.
PROCEDURE
Percutaneous coronary intervention (PCI) was performed according to current clinical practice standards. The radial and femoral approaches using 6 or 7 Fr catheters were the principal routes of vascular access. All patients were treated with unfractionated heparin (at a dose of 70-100 IU/kg). According to the guidelines, patients with stable angina were preloaded with 300 mg of aspirin and 600 mg of clopidogrel. Patients presenting with ACS were preloaded with 300 mg of aspirin and 60 mg of prasugrel or 180 mg of ticagrelor. Previous guidelines for DES and per hospital policies were used to prescribe DAPT and this was also based on the operator’s instructions.
FOLLOW-UP
Survival status was obtained from municipal civil registries. Follow-up information specifically for hospitalisation and major cardiovascular events was obtained through questionnaires which were mailed to individual patients at one, six, 12 and 18 months after the procedure. In the case of an absent response after a reminder mail, patients were called thereafter or information was gathered from general practitioners or hospitals. Information on DAPT status and the date of stopping the P2Y12 inhibitor was collected. When an exact stop date was available (through questionnaires, pharmacies, general practitioners or hospital letters), that date was used to compute the duration of DAPT. When patients did not exactly recall the precise stop date but instead noted that he or she used DAPT for a period of one year, the duration of DAPT was recorded as 365 days. In the case of a patient writing that he/she had visited the hospital, additional medical records and discharge letters were consulted to check if any event had occurred.
DEFINITIONS
ScT was classified as stent thrombosis (ST) according to the Academic Research Consortium (ARC)16. Scaffold thromboses were reported as either acute (≤24 hours), subacute (1-30 days), late (30-365 days), or very late (>365 days). DAPT termination was defined as the date on which one of the two components of DAPT (aspirin or P2Y12 inhibitor) had been terminated.
ENDPOINTS
The primary endpoint in the DAPT study cohort was the incidence rate of definite or probable ScT beyond six months while the patient either was using DAPT or had terminated DAPT. This time period (six to 18 months) was chosen because we assumed that, based on the healing process, the pathophysiology of scaffold thrombosis in the period between six and 18 months was similar. To investigate the time relation with DAPT in more detail, an additional analysis was performed for the first month after DAPT termination compared to the incidence rate while on DAPT.
STATISTICAL ANALYSIS
Categorical variables are reported as counts and percentages, continuous variables as mean±standard deviation or median (25th-75th percentile). For each time period, the ScT incidence was calculated as the number of events divided by the sum of the follow-up times for each individual. The variable “on DAPT” was computed as the stop date of DAPT minus the date of the index procedure. In case of an ScT while the patient was using DAPT, “on DAPT” was reported as days until the event. “off DAPT” was calculated as 18 months post procedure (or the latest available follow-up date) minus the time period until termination of the P2Y12 inhibitor. In the case of ScT while DAPT was terminated, days off DAPT were computed as follows: date of ScT minus date of DAPT termination. The cumulative incidence of study endpoints was estimated according to the Kaplan-Meier method. Patients lost to follow-up were considered at risk until the date of last contact, at which point they were censored. All statistical tests were patient-based, two-sided and a p-value of <0.05 was considered statistically significant. Statistical analyses were performed using SPSS, Version 21 (IBM Corp., Armonk, NY, USA).
Results
Between September 2012 and January 2015, 808 patients were included in the pooled database. The DAPT study cohort consisted of 685 patients (Figure 1). Survival status in this group was known in 100% and the median follow-up duration was 730 (interquartile range [IQR]: 531.8-923.3) days. The median duration of DAPT was 367 (IQR: 365-398) days, with a range from 180 to 1,237 days. One hundred and thirty (19%) patients had a DAPT duration ranging between six months and one year, and 81% had a DAPT duration of at least 365 days. Eighty-nine patients (12.9%) continued DAPT until the last follow-up. Figure 2A displays the individual duration of DAPT for the patients.
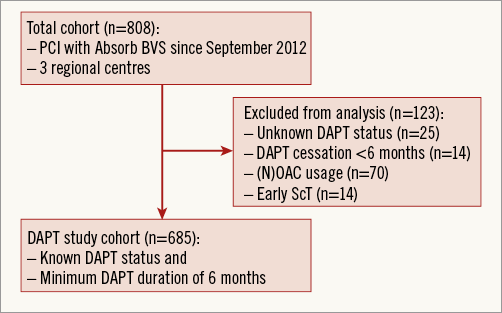
Figure 1. Study flow chart.
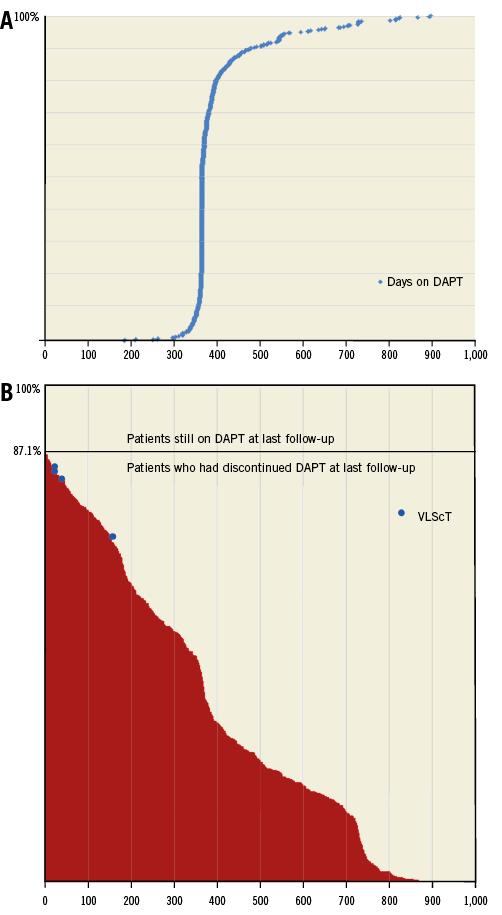
Figure 2. DAPT duration for all indivudual patients. A) Days on DAPT in the DAPT study cohort. B) Days off DAPT in the DAPT study cohort. Blue dots indicate the ScT timing in relation to the number of days off DAPT. VLScT: very late scaffold thrombosis.
BASELINE CHARACTERISTICS
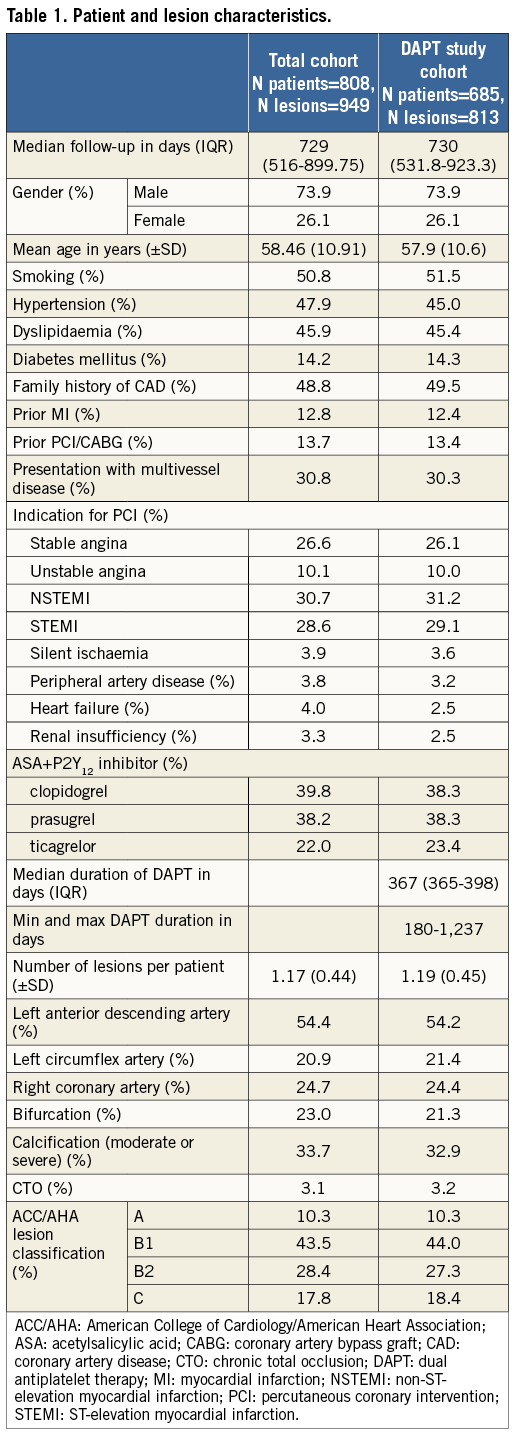
Baseline characteristics of both the full cohort and the DAPT study cohort are presented in Table 1. In the DAPT study cohort, mean age was 57.9 (±10.6) years, 73.9% were male, 14.3% were diabetic, and 12.4% had a history of myocardial infarction. Most patients (70.3%) presented with ACS. The majority of the patients used a potent P2Y12 inhibitor such as prasugrel or ticagrelor (76.6%). The mean number of lesions/patient was 1.19 (±0.45). Moderate or severe lesion calcification, as assessed by angiography, was present in 32.9% and bifurcation in 21.3% of patients. AHA/ACC lesion classification type B2/C was present in 45.7%.
PROCEDURAL DETAILS
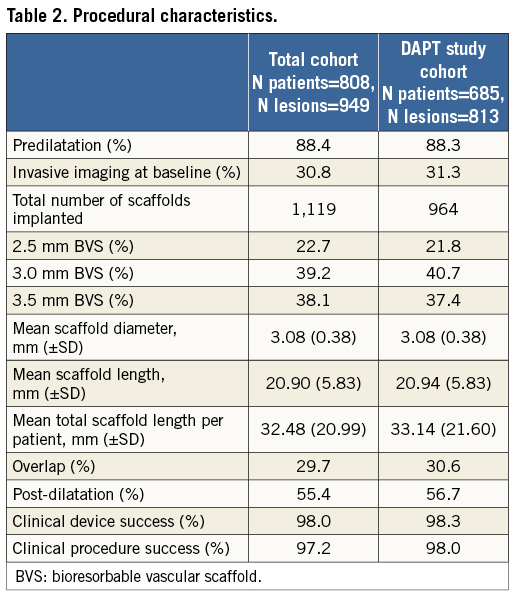
Procedural details are described in Table 2. In the DAPT study cohort, a total of 964 BVS were implanted. Predilatation was performed in 88.3% of the patients, post-dilatation in 56.7% and intravascular imaging (OCT or IVUS) in 31.3%. A 2.5 mm BVS was used in 21.8% of patients. Mean scaffold diameter and mean scaffold length were 3.1 (±0.4) mm and 20.9 (±5.8) mm, respectively. Device success and procedural success were achieved in 98.3% and 98.0%, respectively.
CLINICAL OUTCOMES
In the total cohort of 808 patients, 26 definite or probable ScT occurred with a cumulative event rate (Kaplan-Meier estimate) of 3.3% (95% CI: 2.1-4.5) at 18 months (Figure 3). The majority (1.7%) were early ScT: the acute ScT rate was 0.2% (95% CI: -0.2-0.6) and the subacute ScT rate was 1.5% (95% CI: 0.7-2.3). Late and very late ScT were less frequent: 1.0% (95% CI: 0.2-2.0) and 0.6% (95% CI: 0.02-1.2), respectively. In the DAPT study cohort, Kaplan-Meier estimates for late and very late ScT were similar (0.9% and 0.7%, respectively).
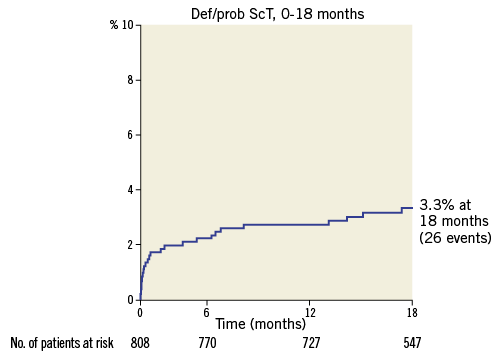
Figure 3. Cumulative ScT rate in the total cohort from the index procedure up to 18 months post procedure. ScT: scaffold thrombosis.
Figure 2B shows the duration in days while off DAPT and the association with very late ScT in the DAPT study cohort. Four cases of very late definite/probable ScT occurred: at 379 days (10 days after DAPT termination), at 416 days (35 days after DAPT termination), at 429 days (20 days after DAPT termination), and at 526 days (149 days after DAPT termination). These cases have been described elsewhere17. The four patients were using aspirin but had terminated P2Y12 inhibitor use. Their duration of DAPT was a little over 365 days. However, this was not based on a specific reason such as an increased ischaemic risk. The rate of definite/probable ScT in this particular time frame was 0.7%.
For reasons of comparability with the current literature, the incidences per 100 patient-years (py) were computed in the DAPT study cohort (Figure 4, Table 3). For calculating the incidence of ScT in the time period six to 18 months, 607.52 py were available and five events occurred (one late ScT and four very late ScT) with an incidence of 0.83/100 py (95% CI: 0.34-1.98).
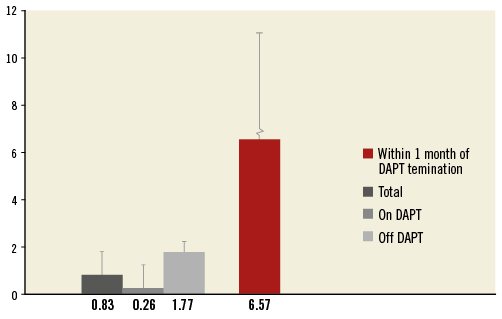
Figure 4. Incidence densities for the whole DAPT study cohort, in patients on and off DAPT and within the first month of termination in the DAPT study cohort. DAPT: dual antiplatelet therapy.
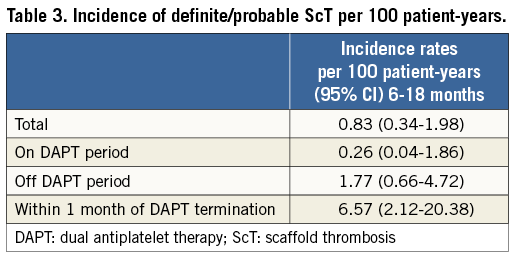
For the period on DAPT, 381.90 py were available and one event occurred (at day 208). This resulted in an incidence of 0.26/100 py (95% CI: 0.04-1.86). For the period after DAPT termination, 225.62 py and four events were reported with an ScT incidence of 1.77/100 py (95% CI: 0.66-4.72), numerically 6.8 times higher than the incidence on DAPT but not statistically significant (p=0.12).
For the incidence of ScT in the first month after DAPT termination, 45.64 py were available and three events occurred, which subsequently provided an incidence of 6.57/100 py (95% CI: 2.12-20.38). This was statistically significant when compared to the incidence in the on DAPT period (p=0.01). The incidence of ScT during the last month of DAPT usage was zero.
Discussion
To the best of our knowledge, this is the first study that reports on the impact of DAPT termination on the occurrence of definite/probable ScT in Absorb BVS in a clearly defined study cohort, reflecting real-world patients. The main findings of our study are as follows: 1) the incidence of definite or probable late and very late ScT in patients who are on DAPT is low; 2) all cases of very late ScT at 18 months were not using DAPT at the time of the event; 3) the incidence of ScT in patients off DAPT is potentially increased within the first 18 months post implantation, with the highest incidence within one month after termination of DAPT.
OVERALL INCIDENCE OF LATE AND VERY LATE ScT
Overall, the late and very late scaffold thrombosis rates in this multicentre, real-world registry were acceptable and comparable to the rates in selected populations as included in approval studies for different countries11,12,15. In this study, and regardless of DAPT status, the overall incidence density of late and very late def/prob ScT was 1.0 and 1.44 per 100 py, respectively. A large all-comers observational cohort study, investigating ST in metallic DES during four-year follow-up reported a late ST incidence density of 0.4 def/prob ST per 100 py in patients treated with newer-generation EES. For SES and PES, incidence densities were higher for both late (SES: 0.7/100 py and PES: 1.5/100 py) and very late ST (SES: 2.8/100 py and PES: 4.0/100 py). In this regard, late and very late ScT incidence in BVS patients seems comparable to first-generation metallic DES18.
DAPT AND LATE EVENTS
At 18 months, there were four patients with VLScT, all while not using DAPT during the event. Three out of four cases appeared to be associated with DAPT termination. The incidence density was 1.79/100 py in patients who were not continuously on DAPT. Importantly, the incidence of ScT within one month of DAPT termination was even higher. In the ABSORB EXTEND study, 50% of the ScT cases were related to either premature DAPT termination or resistance to clopidogrel19. The ABSORB Japan trial has reported two-year follow-up. Two out of four patients with VLScT were not using DAPT at the time of the event. In the recently published ABSORB II RCT, three-year results revealed six cases of VLScT. Of note, all cases of late and very late ScT occurred in patients off DAPT. Moreover, in patients who did not terminate DAPT up to three years, no cases of ScT were described12. In our series, the relationship between the moment of DAPT termination and the occurrence of VLScT was notable, with three out of four cases within 35 days of DAPT termination, a finding not so clear in the ABSORB II and ABSORB Japan trials. Thus, as reported in multiple studies, DAPT termination seems to play an important role in the occurrence of VLScT.
POSSIBLE CAUSES OF LATE ScT
Other factors besides DAPT termination that were associated with ScT were suboptimal implantation technique, late discontinuities, uncovered struts, neoatherosclerosis, high maximum footprint, small minimal lumen diameter, small vessels, higher % diameter stenosis, overlap, ostial lesions and decreased LVEF11,20-25. Late and very late ScT while DAPT was terminated might be explained by the high volume of implanted material, in particular to the increased strut thickness, which could cause laminar flow disturbance and subsequently the triggering of platelet deposition26. This might be a special problem in small vessels or when full dilatation was not achieved without high-pressure post-dilatation using non-compliant balloons. In early BVS registries, there was a higher risk of malapposition, often induced by undersizing, which occurs regularly5. During the first large studies in BVS patients, high-pressure post-dilatation with non-compliant balloons was not mandatory as a result of a case where strut fractures were observed. Nowadays, a different implantation tactic for BVS is used, after an optimal implantation strategy started in January 2014 was associated with a large reduction in ScT incidence20,27. Also, thinner-strut BVS are currently being developed, which will mitigate the risk of ScT.
Study limitations
This was a retrospective and registry data-pooled study. As the sample size is limited and the numbers of this low-frequency event are small, these results should be interpreted with caution and considered hypothesis-generating. More data and dedicated studies are needed to confirm our suggestion to prolong DAPT in BVS-treated patients. Lastly, quantitative coronary analysis (QCA) was not available in all patients.
Conclusions
The incidence of probable/definite late and very late ScT in BVS patients who were on DAPT in our study was low. However, the incidence of early ScT and also the occurrence of very late ScT was not negligible. Between six and 18 months, the incidence of ScT in patients who terminated DAPT was potentially increased.
| Impact on daily practice As long as studies with an optimal implantation strategy have not revealed data on safe DAPT termination before 18 months, it would be reasonable to consider extension of DAPT. Prolonging DAPT even up to three years could be a possible solution in patients with an increased risk of ischaemic events and low bleeding risk (the DAPT score can be used for risk assessment28), as the resorption process of the Absorb BVS is completed in three years and, until that time, the polymer is still present, and the risk of very late ScT is lurking. The decision as to whether or not to continue DAPT beyond a certain time point cannot be made according to a “one size fits all” principle but should be based on each individual patient. |
Acknowledgements
We wish to thank Isabella Kardys for her valuable input and role in event adjudication. We wish to thank Ester Salentijn, Nienke van Ditzhuijzen and Saskia Wemelsfelder for their contribution to data management.
Funding
This research was possible through funding from Abbott Vascular to the Erasmus Medical Center as coordinating centre.
Conflict of interest statement
R. van Geuns, P. Smits, G. Vlachojannis, Y. Onuma, and A. IJsselmuiden have received fees or grants from Abbott Vascular. The other authors have no conflicts of interest to declare.

