Abstract
BACKGROUND: The clinical benefits of optical frequency domain imaging (OFDI)-guided percutaneous coronary intervention (PCI) for acute coronary syndrome (ACS) remain unclear.
AIMS: We sought to compare intravascular ultrasound (IVUS)- and OFDI-guided PCI in patients with ACS.
METHODS: OPINION ACS is a multicentre, prospective, randomised, non-inferiority trial that compared OFDI-guided PCI with IVUS-guided PCI using current-generation drug-eluting stents in ACS patients (n=158). The primary endpoint was in-stent minimum lumen area (MLA), assessed using 8-month follow-up OFDI.
RESULTS: Patients presented with ST-segment elevation myocardial infarction (55%), non-ST-segment elevation myocardial infarction (29%), or unstable angina pectoris (16%). PCI procedural success was achieved in all patients, with comparably low periprocedural complications rates in both groups. Immediately after PCI, the minimum stent area (p=0.096) tended to be smaller for OFDI versus IVUS guidance. Proximal stent edge dissection (p=0.012) and irregular protrusion (p=0.03) were significantly less frequent in OFDI-guided procedures than in IVUS-guided procedures. Post-PCI coronary flow, assessed using corrected Thrombolysis in Myocardial Infarction frame counts, was significantly better in the OFDI-guided group than in the IVUS-guided group (p<0.001). The least squares mean (95% confidence interval [CI]) in-stent MLA at 8 months was 4.91 (95% CI: 4.53-5.30) mm2 and 4.76 (95% CI: 4.35-5.17) mm2 in the OFDI- and IVUS-guided groups, respectively, demonstrating the non-inferiority of OFDI guidance (pnon-inferiority<0.001). The average neointima area tended to be smaller in the OFDI-guided group. The frequency of major adverse cardiac events was similar.
CONCLUSIONS: Among ACS patients, OFDI-guided PCI and IVUS-guided PCI were equally safe and feasible, with comparable in-stent MLA at 8 months. OFDI guidance may be a potential option in ACS patients. This study was registered in the Japan Registry of Clinical Trials (jrct.niph.go.jp: jRCTs052190093).
Recent clinical trials have demonstrated that intravascular ultrasound (IVUS) guidance significantly improves the clinical outcome of percutaneous coronary intervention (PCI)12. Optical frequency domain imaging (OFDI; Lunawave [Terumo Corporation]) is an intravascular imaging device, based on optical coherence tomography (OCT) technology, which enables rapid image acquisition of a long coronary segment (up to 150 mm, 40 mm/s) within a few seconds. Since OFDI has >10 times higher resolution than IVUS, it offers more detailed information on the microstructure during PCI. Several clinical trials demonstrated that OCT-guided PCI is non-inferior to IVUS-guided PCI with regard to clinical outcomes in patients with coronary artery disease34. However, most patients in these trials had chronic coronary syndrome (CCS), and patients with ST-segment elevation myocardial infarction (STEMI) were excluded. Since the culprit plaque of an acute coronary syndrome (ACS) can be more vulnerable and thrombus-rich, the impact of IVUS and OFDI guidance could differ in this setting from that when treating lesions in patients with CCS. Therefore, we conducted the OPINION ACS trial to compare the results of PCI-treated lesions immediately after PCI and at the 8-month follow-up and the clinical outcomes of patients at the 12-month follow-up between OFDI- and IVUS-guided PCI for ACS.
Methods
STUDY DESIGN AND POPULATION
The design of the OPINION ACS trial has been previously described5 (Supplementary Appendix 1, Supplementary Appendix 2). OPINION ACS is a prospective, multicentre, randomised, active-controlled, open-label, parallel-group, non-inferiority trial comparing OFDI-guided PCI with IVUS-guided PCI using current-generation drug-eluting stents (DES). The primary endpoint was in-stent minimum lumen area (MLA) at 8 months (Supplementary Figure 1). Patients admitted to 10 hospitals in Japan between 14 February 2020 and 28 January 2022 were included. An independent data and safety monitoring committee monitored the safety of the trial. The Translational Research Informatics Center (Kobe, Japan) conducted data management, statistical analysis, and site management. OPINION ACS employed the following eligibility criteria: (1) patients aged 20-85 years; (2) ACS patients scheduled for PCI using a current-generation DES to treat a de novo native coronary artery lesion; (3) patients with written informed consent; and (4) patients whose target vessel diameter was 2.25-3.50 mm. ACS includes acute STEMI, non-STEMI (NSTEMI), and unstable angina pectoris. Patients with cardiogenic shock, chronic kidney disease, haemodialysis, or peritoneal dialysis, and female patients who were pregnant or planning to become pregnant were excluded. Eligible patients were then randomly assigned in a 1:1 ratio to receive either OFDI- or IVUS-guided PCI using a web-based randomisation software (conducted at the Translational Research Informatics Center, Kobe, Japan). In this study, we used the minimisation method, which is a dynamic randomisation method that can balance groups with respect to both the numbers in each treatment arm and the characteristics of each group. Randomisation was stratified according to (1) STEMI, (2) history of diabetes, and (3) study site. Participants and investigators were not masked to the allocation.
OPINION ACS was performed according to the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice Guideline as well as according to all applicable Japanese laws, rules, and regulations. The trial protocol was approved by the Institutional Review Board of Kobe University Hospital. All participants provided written informed consent. This study was registered with the Japan Registry of Clinical Trials (jrct.niph.go.jp: jRCTs052190093).
PROCEDURES
Detailed procedures and antithrombotic therapy have been described previously5 and are provided in Supplementary Appendix 3. In the OFDI-guided PCI group, the proximal and distal reference sites were set at cross-sections adjacent to the target lesion that had the most normal appearance and was free of lipidic plaque. The stent diameter was determined by measuring the lumen diameter at the proximal and distal reference sites, and the stent length was determined by measuring the distance from the distal to the proximal reference site. The stent diameter was determined to be 0.25-0.50 mm greater than the mean lumen diameter at the distal reference site.
In the IVUS-guided PCI group, proximal and distal reference sites were set at cross-sections adjacent to the target lesion that had the largest lumen and a plaque burden of <50%. The stent diameter was determined by measuring the vessel diameter (approximated by the external elastic membrane diameter) at the proximal and distal reference sites, and the stent length was determined by measuring the distance from the distal to the proximal reference site. The stent diameter was determined to be equal to or greater than the mean vessel diameter at the distal reference site and smaller than the mean vessel diameter at the proximal reference site.
Use of thrombus aspiration was left to the operator’s discretion. Following stent implantation, acute results were evaluated by iterative intravascular imaging. In cases of incomplete stent expansion (minimum stent area [MSA]/average of proximal and distal reference luminal areas <0.8) or incomplete stent apposition, post-dilation using a balloon with a diameter 0-0.25 mm greater than the mean lumen diameter at the proximal reference site was recommended, if deemed safe and feasible, followed by further intravascular imaging.
In addition to post-PCI imaging using the allocated imaging modality, the OFDI-guided PCI group underwent post-PCI IVUS, whereas the IVUS-guided PCI group underwent post-PCI OFDI. The operators were not blinded to the findings of non-allocated imaging, but no additional procedures were allowed after the final non-allocated imaging. To assess the contrast media volume needed for PCI, we compared the total contrast volume in the OFDI-guided PCI group with that in the IVUS-guided PCI group, excluding the volume needed for the final OFDI image acquisition in the IVUS-guided PCI group. Both groups underwent follow-up angiography with OFDI 8 months after PCI (Supplementary Figure 1).
STUDY OUTCOMES
The primary endpoint of the OPINION ACS study was the MLA in the stented segment, as determined by the 8-month follow-up OFDI. The secondary endpoints are described in the Supplementary Appendix 3.
QUANTITATIVE ANGIOGRAPHY, OPTICAL FREQUENCY DOMAIN IMAGING, AND INTRAVASCULAR ULTRASOUND ANALYSIS
Off-line angiogram, OFDI, and IVUS analyses were performed using dedicated software (QAngio XA [Medis Medical Imaging]; Lunawave [Terumo Corporation]; echoPlaque [INDEC Medical Systems]) in an independent core laboratory (Micron Inc., Osaka, Japan), blinded to the clinical presentation, lesion and procedural characteristics, and randomisation. Stent edge dissection, haematoma, plaque protrusion, and thin-cap fibroatheroma were qualitatively evaluated6789. In-stent tissue protrusion was divided into 3 categories: smooth, disrupted fibrous tissue, and irregular (Supplementary Figure 2)8. All angiographic, OFDI, and IVUS parameters were prespecified by the core laboratory. Details of the OFDI and IVUS analyses are provided in Supplementary Appendix 4.
CLINICAL OUTCOME
Clinical follow-up in the OPINION ACS trial was scheduled at discharge and at 8 and 12 months after PCI to evaluate the incidence of cardiac death, myocardial infarction attributed to the target vessel, ischaemia-driven target lesion revascularisation (TLR), target vessel revascularisation (TVR), target vessel failure (a composite of cardiac death, target vessel-related myocardial infarction, and ischaemia-driven TVR), and major adverse cardiac events (a composite outcome of cardiac death, myocardial infarction, and ischaemia-driven TLR) during the follow-up period (Supplementary Appendix 4). The incidence of distal embolisation during the PCI was also evaluated. The clinical events were adjudicated by each institution. Detailed statistical analyses are described in Supplementary Appendix 3.
Results
PATIENT FLOW
A total of 158 patients were enrolled and randomised to undergo either OFDI-guided (n=79) or IVUS-guided PCI (n=79). Nineteen patients were excluded from the analysis due to registration errors related to the process of obtaining informed consent. Details are described in Figure 1.
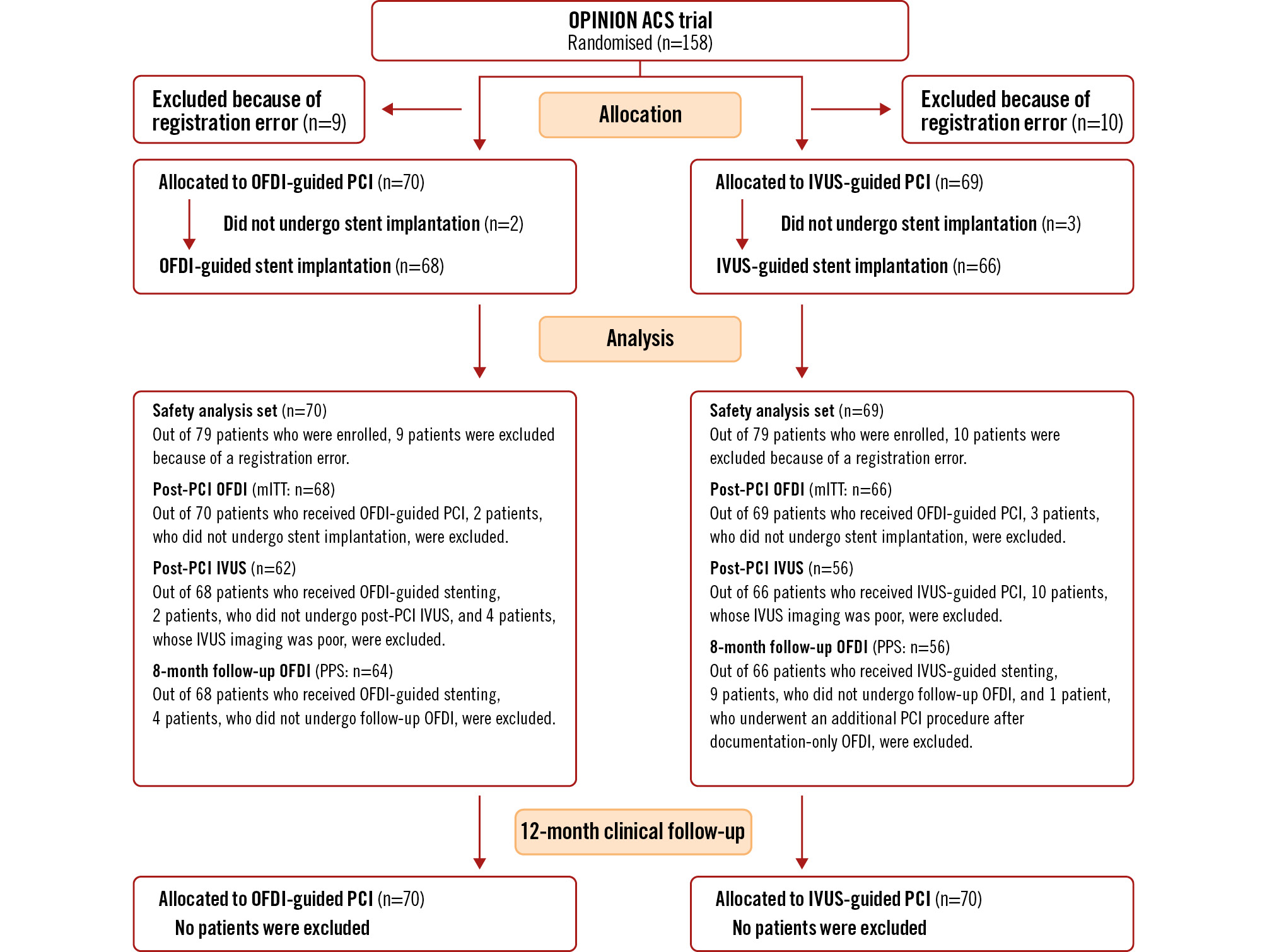
Figure 1. Trial profile of the OPINION ACS study. ACS: acute coronary syndrome; IVUS: intravascular ultrasound; OFDI: optical frequency domain imaging; PCI: percutaneous coronary intervention; mITT: modified intention-to-treat; PPS: per protocol set
COMPARISON OF BASELINE AND PROCEDURAL CHARACTERISTICS IN THE OFDI-GUIDED AND IVUS-GUIDED PERCUTANEOUS CORONARY INTERVENTION GROUPS
Patient clinical characteristics were well balanced between the OFDI-guided and IVUS-guided PCI groups (Table 1, Supplementary Table 1). Most patients had STEMI (55%), followed by NSTEMI (29%) or unstable angina pectoris (16%). Angiographic lesion characteristics pre-PCI showed no significant differences between the groups, except for different distributions of target vessels (Table 1, Supplementary Table 1-Supplementary Table 3).
Table 2 and Supplementary Table 4 summarise the procedural characteristics. The OFDI-guided PCI group showed a trend towards a smaller stent diameter and shorter total stent length than the IVUS-guided PCI group. The prevalence of distal protection device use was significantly lower in the OFDI-guided PCI group than in the IVUS-guided PCI group. The maximum balloon diameter was significantly smaller in the OFDI-guided PCI group than in the IVUS-guided PCI group. Based solely on the OFDI/IVUS evaluation, additional PCI procedures for lesion preparation before stenting and PCI optimisation after stenting were performed in 41 (63.1%) out of 68 patients in the OFDI-guided PCI group and in 40 (66.7%) out of 66 patients in the IVUS-guided PCI group (p=0.710).
The total fluoroscopic time was significantly shorter in the OFDI-guided PCI group than in the IVUS-guided PCI group. The amount of contrast medium used for PCI was significantly greater in the OFDI-guided PCI group than in the IVUS-guided PCI group. PCI procedural success was achieved in all cases (Table 2). There were no OFDI/IVUS procedure-related complications in either group. Antithrombotic medications at discharge were not significantly different between the 2 groups (Supplementary Table 5).
Table 1. Baseline characteristics (modified intention-to-treat).
| OPINION ACS (modified intention-to-treat: n=134) | |||
|---|---|---|---|
| OFDI-guided (n=68) |
IVUS-guided (n=66) |
p-value | |
| Age, years | 65.6±11.6 | 66.3±9.5 | 0.975 |
| Male | 52 (76.5) | 58 (87.9) | 0.114 |
| Coronary risk factors | |||
| Hypertension | 47 (69.1) | 37 (56.1) | 0.153 |
| Dyslipidaemia | 44 (64.7) | 40 (60.6) | 0.721 |
| Diabetes mellitus | 25 (36.8) | 23 (34.8) | 0.858 |
| Current smoker | 9 (13.2) | 5 (7.6) | 0.385 |
| Family history of CAD | 6 (8.8) | 8 (12.1) | 0.582 |
| Obesity | 19 (27.9) | 17 (25.8) | 0.846 |
| Prior myocardial infarction | 1 (1.5) | 5 (7.6) | 0.112 |
| Prior PCI | 4 (5.9) | 9 (13.6) | 0.153 |
| Prior CABG | 0 (0) | 1 (1.5) | 0.492 |
| Clinical presentation | 1.000 | ||
| STEMI | 38 (55.9) | 35 (53.0) | 0.971 |
| NSTEMI | 19 (27.9) | 20 (30.3) | |
| Unstable angina pectoris | 11 (16.2) | 11 (16.7) | |
| Lesion characteristics | |||
| Target coronary artery | 0.010 | ||
| LAD | 40 (58.8) | 25 (37.9) | |
| LCx | 11 (16.2) | 8 (12.1) | |
| RCA | 17 (25.0) | 33 (50.0) | |
| Location | 0.968 | ||
| Proximal | 23 (33.8) | 24 (36.4) | |
| Mid | 37 (54.4) | 34 (51.5) | |
| Distal | 8 (11.8) | 8 (12.1) | |
| ACC/AHA lesion type | 0.062 | ||
| A, B1 | 42 (61.8) | 51(77.3) | |
| B2, C | 26 (38.2) | 15 (22.7) | |
| Total occlusion | 28 (41.2) | 25 (37.9) | 0.726 |
| Ulceration | 3 (4.4) | 3 (4.5) | 1.000 |
| Moderate or heavy calcification | 4 (5.9) | 3 (4.5) | 1.000 |
| Thrombus | 25 (36.8) | 29 (43.9) | 0.481 |
| Data are mean±standard deviation or n (%). ACC/AHA: American College of Cardiology/American Heart Association; CABG: coronary artery bypass graft; CAD: coronary artery disease; IVUS: intravascular ultrasound; LAD: left anterior descending artery; LCx: left circumflex artery; NSTEMI: non-ST-segment elevation myocardial infarction; OFDI: optical frequency domain imaging; PCI: percutaneous coronary intervention; RCA: right coronary artery; STEMI: ST-segment elevation myocardial infarction | |||
Table 2. Procedural characteristics and periprocedural complications (modified intention-to-treat).
| OPINION ACS (modified intention-to-treat: n=134) | |||
|---|---|---|---|
| OFDI-guided (n=68) |
IVUS-guided (n=66) |
p-value | |
| Stent and procedures | |||
| Stent diameter, mm | 3.0 (2.8-3.5) | 3.0 (3.0-3.5) | 0.053 |
| Total stent length, mm | 28.0 (21.0-33.0) | 28.0 (24.0-35.0) | 0.087 |
| Number of stents per patient | 1.0 (1.0-1.0) | 1.0 (1.0-1.0) | 0.461 |
| Stent type | 0.485 | ||
| Ultimaster sirolimus-eluting stent1 | 63 (92.7) | 57 (86.4) | |
| Everolimus-eluting stent | 4 (5.9) | 5 (7.6) | |
| Resolute Onyx2 | 0 (0) | 1 (1.5) | |
| Orsiro3 | 1 (1.5) | 3 (4.6) | |
| Others | 0 (0) | 0 (0) | |
| Thrombus aspiration | 25 (36.2) | 23 (34.9) | 1.000 |
| Rotablation | 0 (0) | 2 (3.0) | 0.237 |
| Distal protection | 3 (4.4) | 11 (16.7) | 0.023 |
| Post-dilation | 48 (69.6) | 40 (60.6) | 0.285 |
| Maximum balloon diameter, mm | 3.3 (3.0-3.5) | 3.5 (3.0-4.0) | 0.030 |
| Maximum inflation pressure, atm | 14.0 (12.0-16.0) | 14.0 (12.0-18.0) | 0.528 |
| Further intervention after imaging | |||
| Predilation with upsized balloon | 17 (25.0) | 19 (28.8) | 0.698 |
| Predilation at high pressure | 8 (11.8) | 5 (7.6) | 0.561 |
| Predilation with cutting balloon | 7 (10.3) | 4 (6.1) | 0.531 |
| Rotablation | 0 (0) | 0 (0) | NA |
| Post-dilation | 23 (33.8) | 21 (31.8) | 0.855 |
| Additional stenting | 1 (1.5) | 3 (7.6) | 0.361 |
| Total fluoroscopic time, min | 26.0 (19.5-37.0) | 33.0 (23.0-46.0) | 0.046 |
| Amount of contrast needed for PCI, ml | 144.0 (116.0-180.0) | 118.0 (94.0-140.0) | <0.001 |
| PCI procedural success | 68 (100) | 66 (100) | NA |
| Periprocedural complications | |||
| Life-threatening arrhythmia | 3 (4.3) | 3 (4.5) | 1.000 |
| Coronary dissection | 1 (1.4) | 2 (3.0) | 0.613 |
| Coronary spasm | 1 (1.4) | 1 (1.5) | 1.000 |
| Distal embolisation | 3 (4.3) | 7 (10.6) | 0.200 |
| Side branch occlusion | 4 (5.8) | 3 (4.5) | 1.000 |
| Air emboli | 0 (0) | 0 (0) | NA |
| Acute coronary occlusion | 0 (0) | 0 (0) | NA |
| Periprocedural complications due to imaging modalities | 0 (0) | 0 (0) | NA |
| Data are median (interquartile range) or n (%). 1by Terumo; 2by Medtronic; 3by Biotronik. IVUS: intravascular ultrasound; NA: not applicable; OFDI: optical frequency domain imaging; PCI: percutaneous coronary intervention | |||
OFDI AND IVUS FINDINGS POST-PERCUTANEOUS CORONARY INTERVENTION
The MSA and mean stent area measured by OFDI tended to be smaller, while the frequency of malapposed struts tended to be higher in the OFDI-guided PCI group than in the IVUS-guided PCI group; however, the malapposed area and volume were comparable between the 2 groups (Table 3).
Overall, the total tissue protrusion volume was significantly smaller in the OFDI-guided PCI group than in the IVUS-guided PCI group (Table 3). Specifically, the prevalence and volume of irregular protrusions were significantly smaller in the OFDI-guided PCI group than in the IVUS-guided PCI group.
Post-PCI IVUS showed that, in adjacent reference segments, the percentage plaque volume and the frequency of lesions with residual stent edge plaque burden >50% were comparable between the groups (Supplementary Table 6). Post-PCI OFDI showed that the frequency of proximal stent edge dissection was significantly lower in the OFDI-guided PCI group than in the IVUS-guided PCI group. The occurrence of haematoma was rare and comparable in both groups (Table 3, Supplementary Table 6).
Table 3. OFDI results post-PCI (modified intention-to-treat).
| OPINION ACS (modified intention-to-treat: n=134) | |||
|---|---|---|---|
| OFDI-guided (n=68) |
IVUS-guided (n=66) |
p-value | |
| In-stent segment | |||
| 3D analysis | |||
| Stent volume, mm3 | 190.49 [144.07-232.75] | 217.13 [164.99-288.57] | 0.018 |
| Stent volume index, mm3/mm | 6.77 [5.69-8.88] | 7.69 [6.35-8.97] | 0.071 |
| Lumen volume, mm3 | 187.32 [141.21-224.41] | 217.57 [167.52-284.77] | 0.024 |
| Lumen volume index, mm3/mm | 6.78 [5.70-8.80] | 7.74 [6.29-8.98] | 0.077 |
| Cross-section-based analysis | |||
| Length, mm | 27.62±10.79 | 30.51±12.28 | 0.124 |
| Mean stent area*, mm2 | 7.26 (6.72-7.80) | 7.93 (7.38-8.48) | 0.090 |
| Minimum stent area, mm2 | 5.56 [4.51-7.66] | 6.33 [4.98-7.75] | 0.096 |
| Mean lumen area*, mm2 | 7.21 (6.70-7.72) | 7.80 (7.28-8.31) | 0.112 |
| Minimum lumen area, mm2 | 5.18 [4.32-7.03] | 5.69 [4.85-7.37] | 0.086 |
| Stent expansion index* | 0.90 (0.78-1.00) | 0.88 (0.76-1.06) | 0.772 |
| Cases with optimal stent expansion | 45 (66.2) | 37 (56.1) | 0.335 |
| Stent malapposition | |||
| Malapposed strut*, % | 3.66 (2.58-5.17) | 2.31 (1.60-3.33) | 0.072 |
| Stent malapposition volume, mm3 | 1.86 [0.79-4.82] | 1.88 [0.58-4.10] | 0.690 |
| Mean stent malapposition area*, mm2 | 0.13 (0.10-0.16) | 0.14 (0.10-0.17) | 0.754 |
| Tissue protrusion | |||
| Quantitative tissue protrusion analysis | |||
| Tissue protrusion volume, mm3 | 6.47 [4.17-12.24] | 8.37 [5.57-16.45] | 0.037 |
| Mean tissue protrusion area*, mm2 | 0.33 (0.22-0.43) | 0.41 (0.30-0.51) | 0.276 |
| Classification of tissue protrusion | |||
| Smooth protrusion | 35 (51.5) | 28 (42.4) | 0.305 |
| Disrupted protrusion | 27 (39.7) | 13 (19.7) | 0.014 |
| Irregular protrusion | 58 (85.3) | 64 (97.0) | 0.030 |
| Quantitative irregular protrusion analysis | |||
| Number of irregular protrusions | 1.50 [1.00-2.00] | 2.00 [2.00-3.00] | <0.001 |
| Irregular protrusion volume, mm3 | 2.43 [0.87-4.78] | 4.22 [1.91-10.40] | 0.004 |
| Mean irregular protrusion area*, mm2 | 0.41 (0.33-0.50) | 0.45 (0.38-0.53) | 0.470 |
| Max irregular protrusion length, mm | 6.14 [4.05-11.39] | 12.09 [7.09-17.22] | <0.001 |
| Max irregular protrusion area, mm2 | 0.72 [0.38-1.18] | 0.79 [0.48-1.53] | 0.099 |
| Max irregular protrusion thickness, mm | 0.40 [0.27-0.51] | 0.45 [0.33-0.68] | 0.062 |
| Stent edge dissection and haematoma | |||
| Overall stent edge dissection | 8 (11.8) | 15 (22.7) | 0.111 |
| Proximal edge dissection | 3 (4.7) | 12 (20) | 0.012 |
| Distal edge dissection | 5 (7.4) | 8 (12.5) | 0.388 |
| Haematoma | 2 (2.9) | 2 (3.0) | 1.000 |
| Proximal reference segment | |||
| Length, mm | 5.06 [5.06-5.06] | 5.06 [4.94-5.06] | 0.497 |
| Lumen volume, mm3 | 38.58 [25.25-51.18] | 39.23 [28.94-47.09] | 0.823 |
| Mean lumen area*, mm2 | 7.21 (6.70-7.72) | 7.80 (7.28-8.31) | 0.112 |
| Minimum lumen area, mm2 | 6.71 [4.84-9.53] | 7.17 [5.24-9.38] | 0.450 |
| Distal reference segment | |||
| Length, mm | 5.06 [5.06-5.06] | 5.06 [5.06-5.06] | 0.945 |
| Lumen volume, mm3 | 25.91 [19.42-37.81] | 29.71 [20.58-41.55] | 0.137 |
| Mean lumen area*, mm2 | 5.19 (3.73-7.51) | 5.73 (3.85-7.88) | 0.282 |
| Minimum lumen area, mm2 | 4.39 [2.82-6.49] | 4.75 [3.44-6.85] | 0.420 |
| Data are n (%) or median [interquartile range] for crude analysis and mean (95% confidence interval) for multilevel analysis. *assessed by multilevel analysis. Stent volume index indicates stent volume/length. Cases with optimal stent expansion indicate those with a stent expansion index >0.8. 3D: three-dimensional; IVUS: intravascular ultrasound; OFDI: optical frequency domain imaging; PCI: percutaneous coronary intervention | |||
THROMBOLYSIS IN MYOCARDIAL INFARCTION FLOW ANALYSIS
Post-PCI angiographic analysis revealed that the prevalence of Thrombolysis in Myocardial Infarction (TIMI) flow grade 3 was significantly higher in the OFDI-guided PCI group than in the IVUS-guided PCI group (Supplementary Table 2, Figure 2). Coronary flow after PCI, as assessed by corrected TIMI frame counts, was significantly better in the OFDI-guided PCI group than in the IVUS-guided PCI group (Figure 2). There was a significant positive relationship between the corrected TIMI frame count and the volume of irregular protrusion on post-PCI OFDI.
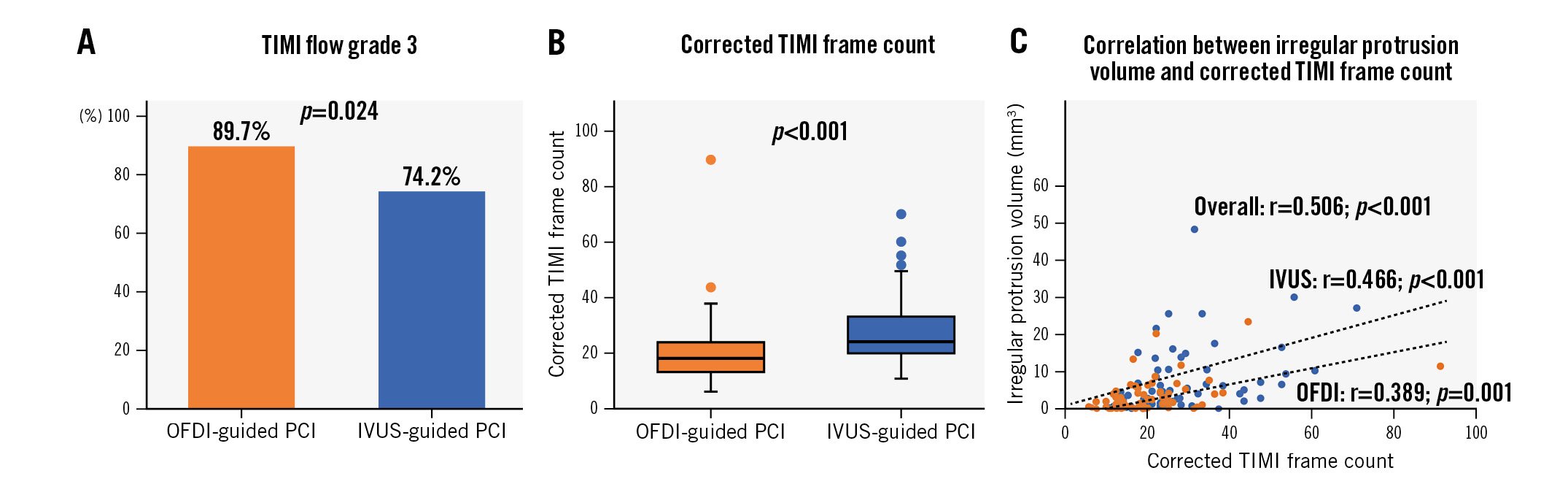
Figure 2. TIMI flow analysis. A) TIMI flow grade 3. B) Corrected TIMI frame count. C) Correlation between irregular protrusion volume and corrected TIMI frame count. IVUS: intravascular ultrasound; OFDI: optical frequency domain imaging; PCI: percutaneous coronary intervention; TIMI: Thrombolysis in Myocardial Infarction
COMPARISON OF 8-MONTH OPTICAL FREQUENCY DOMAIN IMAGING FINDINGS
The primary endpoint, the least square means (95% confidence interval [CI]) of MLA by OFDI at 8 months, was 4.91 (95% CI: 4.53-5.30) mm2 in the OFDI-guided PCI group and 4.76 (95% CI: 4.35-5.17) mm2 in the IVUS-guided PCI group (Supplementary Table 7). MLA with OFDI guidance was non-inferior to IVUS guidance (1-sided 95% lower CI: −0.31 mm2; p<0.001) (Central illustration). This result was similar to that observed in the modified intention-to-treat population (Supplementary Table 8).
The mean neointimal volume and area were significantly smaller in the OFDI-guided PCI group than in the IVUS-guided PCI group (Table 4). The changes in MLA and mean lumen area from post-PCI to 8-month follow-up OFDI were significantly smaller in the OFDI-guided PCI group than in the IVUS-guided PCI group (MLA change: −0.92 [95% CI: −1.35 to −0.19] mm2 vs −1.19 [95% CI: −1.84 to −0.80] mm2; p=0.006; average lumen area change: −0.79 [95% CI: −1.25 to 0.37] mm2 vs −1.14 [95% CI: −1.63 to −0.58] mm2; p=0.020). No statistical differences in the mean lumen volume or area at 8-month follow-up were noted between the groups.
The frequency of malapposed struts on 8-month follow-up OFDI was similar between the 2 groups. The frequency of uncovered struts was low in both groups but significantly lower in the IVUS-guided PCI group than in the OFDI-guided PCI group. Most of the dissections detected post-PCI had healed; the stent edge dissection remained in 1 reference segment in each group (Table 4).
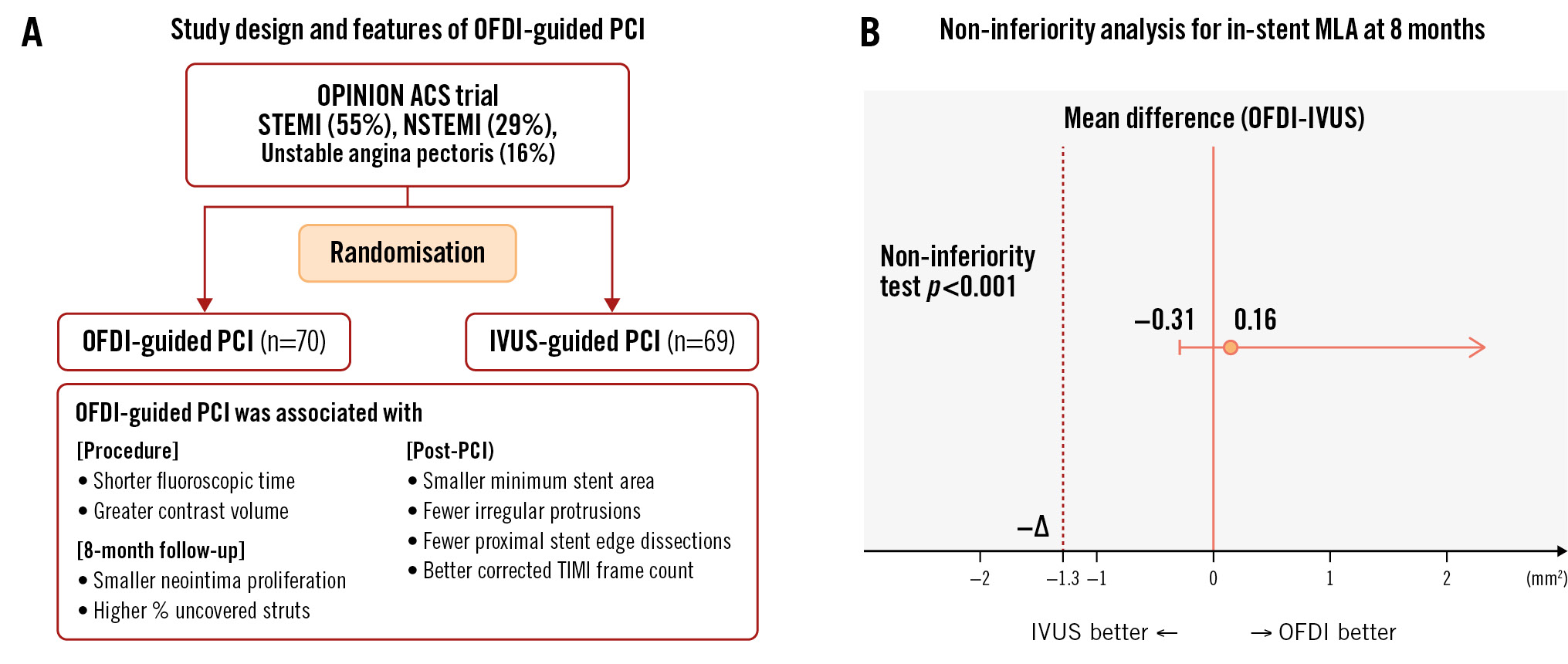
Central illustration. OFDI versus IVUS in percutaneous coronary intervention for acute coronary syndrome. A) OPINION ACS: study design and features of OFDI-guided PCI. B) Primary endpoint result. ACS: acute coronary syndrome; IVUS: intravascular ultrasound; MLA: minimum lumen area; NSTEMI: non-ST-segment elevation myocardial infarction; OFDI: optical frequency domain imaging; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction; TIMI: Thrombolysis in Myocardial Infarction
Table 4. OFDI results at 8-month follow-up (per protocol set).
| OPINION ACS (per protocol set: n=120) | |||
|---|---|---|---|
| OFDI-guided (n=64) | IVUS-guided (n=56) | p-value | |
| In-stent segment | |||
| 3D analysis | |||
| Stent volume, mm3 | 192.81 [140.74-236.00] | 211.97 [158.58-276.17] | 0.087 |
| Stent volume index, mm3/mm | 6.83 [5.74-9.07] | 7.81 [5.95-8.96] | 0.405 |
| Lumen volume, mm3 | 168.65 [108.76-209.26] | 177.05 [132.51-233.91] | 0.204 |
| Lumen volume index, mm3/mm | 5.99 [4.89-8.07] | 6.41 [5.02-7.50] | 0.628 |
| Neointima volume, mm3 | 24.73 [14.31-35.12] | 33.21 [20.45-47.12] | 0.016 |
| Cross-section-based analysis | |||
| Length, mm | 25.25 [19.49-32.15] | 28.35 [23.67-35.19] | 0.063 |
| Mean stent area*, mm2 | 7.33 (6.79-7.88) | 7.86 (7.31-8.41) | 0.182 |
| Minimum stent area, mm2 | 5.67 [4.65-8.15] | 5.97 [5.07-7.45] | 0.529 |
| Mean lumen area*, mm2 | 6.39 (5.88-6.90) | 6.65 (6.13-7.18) | 0.479 |
| Minimum lumen area, mm2 | 4.40 [3.34-6.44] | 4.72 [3.79-5.70] | 0.891 |
| Mean stent eccentricity index* | 0.93 (0.92-0.94) | 0.92 (0.92-0.93) | 0.050 |
| Mean neointima area*, mm2 | 1.03 (0.92-1.13) | 1.24 (1.13-1.35) | 0.007 |
| Mean neointima thickness*, µm | 125 (95-154) | 138 (113-182) | 0.069 |
| Stent malapposition | |||
| Stent with malapposition | 16 (25.0) | 9 (16.1) | 0.265 |
| Malapposed strut*, % | 0.16 (0.09-0.27) | 0.10 (0.05-0.17) | 0.184 |
| Stent malapposition volume, mm3 | 0.45 [0.09-1.54] | 0.36 [0.10-1.48] | 0.650 |
| Mean stent malapposition area*, mm2 | 0.06 (0.03-0.09) | 0.04 (0.01-0.07) | 0.425 |
| Uncovered struts*, % | 1.54 (1.05-2.26) | 0.83 (0.54-1.27) | 0.031 |
| Proximal reference segment | |||
| Length, mm | 5.06 [5.06-5.06] | 5.06 [5.06-5.06] | 0.387 |
| Lumen volume, mm3 | 35.13 [25.61-47.81] | 35.75 [24.49-46.38] | 0.928 |
| Mean lumen area*, mm2 | 8.20 (7.32-9.07) | 8.17 (7.27-9.08) | 0.969 |
| Minimum lumen area, mm2 | 6.23 [4.60-8.56] | 6.73 [3.84-7.88] | 0.759 |
| Dissection | 1 (1.6) | 0 (0) | 1.000 |
| Distal reference segment | |||
| Length, mm | 5.06 [5.06-5.06] | 5.06 [5.06-5.06] | 0.148 |
| Lumen volume, mm3 | 26.08 [20.13-40.02] | 27.60 [20.63-41.29] | 0.629 |
| Mean lumen area*, mm2 | 5.96 (5.33-6.59) | 6.16 (5.51-6.81) | 0.664 |
| Minimum lumen area, mm2 | 4.56 [3.52-6.63] | 4.74 [3.11-6.99] | 0.711 |
| Dissection | 0 (0) | 1 (1.8) | 0.466 |
| Data are n (%) or median [interquartile range] for crude analysis and mean (95% confidence interval) for multilevel analysis. *assessed by multilevel analysis. Volume index indicates volume/length. 3D: three-dimensional; IVUS: intravascular ultrasound; OFDI: optical frequency domain imaging | |||
CLINICAL OUTCOMES
The rates of cardiac death, myocardial infarction, target vessel-related myocardial infarction, ischaemia-driven TVR, ischaemia-driven TLR, major adverse cardiac events, stent thrombosis, and stroke were similar between the groups (Supplementary Table 9). Details of the cases with ischaemia-driven TVR are shown in Supplementary Figure 3. The incidence of contrast-induced nephropathy did not differ between the groups (Supplementary Table 9).
Discussion
This study is the first prospective randomised trial to compare the impact of IVUS and OFDI guidance in patients undergoing PCI for ACS, with an evaluation of midterm arterial responses using 8-month OFDI and 12-month clinical outcomes. The main findings of this study are as follows. 1) Both OFDI-guided and IVUS-guided PCI achieved high procedural success rates with low and comparable periprocedural complication rates. 2) Post-PCI, the OFDI-guided PCI group showed a trend of a smaller MSA with significantly fewer proximal stent-edge dissections and irregular protrusions than the IVUS-guided PCI group. The OFDI-guided PCI group achieved a significantly higher prevalence of TIMI flow grade 3 with a better corrected TIMI frame count post-PCI. 3) The primary endpoint of MLA, assessed by 8-month follow-up OFDI, demonstrated the non-inferiority of OFDI guidance due to less neointimal hyperplasia in the OFDI-guided PCI group.
Ensuring safety is crucial in invasive imaging-guided PCI, particularly for ACS patients. A recent multicentre, randomised trial comparing IVUS-guided PCI to angiography-guided PCI in ACS patients demonstrated that IVUS-guided PCI significantly reduced the incidence of target vessel failure at 1 year post-PCI when compared with angiography-guided PCI, with comparable safety endpoint results between the 2 groups10. Unlike IVUS, OFDI requires intracoronary contrast medium injections, raising concerns about potential periprocedural adverse events. However, in this study, the rate of procedure-related complications in OFDI-guided PCI was notably low and comparable to that in IVUS-guided PCI. Although the OFDI-guided PCI group used greater amounts of contrast for PCI, the incidence of contrast-induced nephropathy post-PCI was similar between the 2 groups. Interestingly, the total fluoroscopic time was significantly shorter in the OFDI-guided PCI group than in the IVUS-guided PCI group. Although speculative, a faster OFDI pullback speed and angiographic coregistration with automatic measurement may have contributed to simplifying the PCI procedure, resulting in a shorter procedural time.
Post-PCI MSA is a robust predictor of TLR and stent thrombosis following stent implantation. Previous studies consistently highlighted that intravascular imaging-guided PCI using OCT or IVUS achieves a significantly larger MSA than angiography-guided PCI. However, the relationship between stent expansion and imaging modalities such as OCT and IVUS has been controversial. In our prior OPINION trial, we reported that the selected stent diameter was slightly but significantly smaller in the OFDI-guided PCI group than in the IVUS-guided PCI group, resulting in a smaller post-PCI MSA in the OFDI-guided PCI group11. The OCTIVUS trial echoed these results, demonstrating that the selected maximum stent and balloon sizes were notably larger in the IVUS group than in the OCT group4. Conversely, the ILUMIEN III trial, which employed an alternative OCT algorithm for optimal stent selection based on the external elastic lamina diameter, showed similar post-PCI MSA between OCT and IVUS guidance12. In the current study, operators were guided to determine the stent diameter based on the distal lumen diameter for the OFDI-guided PCI group and the vessel diameter for the IVUS-guided PCI group. Consequently, the OFDI-guided PCI group exhibited a slightly smaller stent and maximum balloon diameter than the IVUS-guided PCI group, leading to a trend toward a smaller MSA post-PCI. However, these subtle differences did not translate into significant variations in MLA in the stented segment at 8-month follow-up OFDI, in angiographic parameters, or in binary restenosis rates at 8-month follow-up, probably due to less neointimal proliferation in the OFDI-guided PCI group.
Attaining a large MSA can have inherent complexities. The recent OCTIVUS randomised clinical trial reported a lower incidence of major procedural complications (e.g., major dissection, coronary perforation, vasospasm, thrombus formation, air embolisation, slow flow or no reflow, distal embolisation, acute closure, ventricular arrhythmia, cardiac tamponade, or cardiogenic shock) in the OCT-guided PCI group than in the IVUS-guided PCI group4. Although the precise reasons for these differences remain unclear, speculation points to a potentially more aggressive interventional approach in the IVUS arm, including larger stents and balloon sizing. Consistently, OFDI-guided PCI in our current study utilised smaller stent sizing and selected smaller maximum balloon sizes; this approach was associated with a lower incidence of stent edge dissection at the proximal edge, a higher incidence of cases achieving TIMI grade 3 flow with a better corrected TIMI frame count, and a numerically decreased occurrence of distal embolisation compared with IVUS-guided PCI.
Regarding the stent edge dissection, the OFDI-guided PCI group had significantly fewer proximal stent edge dissections than the IVUS-guided PCI group. Previous investigations, such as the ILUMIEN III trial12, observed a significantly lower prevalence of post-PCI dissection in the OCT-guided PCI group than in the IVUS- and angiography-guided PCI groups. In our previous OPINION imaging substudy11, the incidence of stent edge dissection with haematoma, a more severe form of stent edge dissection, was notably higher in the IVUS-guided PCI group than in the OFDI-guided PCI group, particularly at the proximal stent edges. Although still speculative, the differences in stent and post-balloon sizing may have contributed to the lower prevalence of stent edge dissections observed in the OFDI-guided PCI group. Considering the potential impact of stent edge-related issues, particularly in patients with haemodynamically unstable ACS, a smooth stent landing facilitated by OFDI guidance emerges as a positive aspect of OFDI-guided PCI.
In our study, the OFDI-guided PCI group demonstrated a significantly lower corrected TIMI frame count after PCI than the IVUS-guided PCI group. Amano et al reported a higher incidence of slow flow after stent implantation in lesions with irregular protrusions13; our study results align with this finding. We also found a statistically significant association between the volume of irregular protrusions and corrected TIMI frame count post-PCI. In our study, we observed a significantly lower incidence of irregular protrusions in the OFDI-guided PCI group than in the IVUS-guided PCI group post-PCI. Larger stent sizing with a selection of larger maximum balloon sizes might accelerate the development of irregular protrusions through the stent struts, potentially leading to distal embolisation during PCI. These results are particularly noteworthy in PCI for ACS patients, where no-reflow/slow-flow phenomena are more likely to occur.
In our study, despite a smaller post-PCI MSA in the OFDI-guided PCI group, we found comparable 8-month MLAs between the 2 groups. We attribute this to potential factors such as greater in-stent protrusion post-PCI and differences in neointimal progression. In addition, the OFDI-guided PCI group exhibited smaller neointima proliferation than the IVUS-guided PCI group. Similar trends were observed in a previous OPINION imaging substudy involving patients with CCS11. Previous studies in animals and humans have consistently demonstrated a positive relationship between vascular injury severity and increased neointimal proliferation141516. This relationship has been observed not only in lesions treated with bare metal stents17 but also in those treated with DES16. We speculate that the inflammatory reaction triggered to repair local arterial injury induced by IVUS-guided large stent and balloon sizing might have led to accelerated reactive neointimal growth within the stents. These findings support our speculation that IVUS-guided PCI may contribute to increased neointimal hyperplasia due to more aggressive stent sizing compared to OFDI-guided PCI. Considering the lower prevalence of stent edge dissection and the lower corrected TIMI frame count with comparable 8-month MLAs, opting for a smooth stent landing by adjusting the stent expansion to the lumen size under OFDI guidance might emerge as a potential option for guiding PCI in patients with ACS.
Limitations
First, the small sample size may have increased the possibility of selection bias, and the study was not sufficiently powered to evaluate the clinical impact of imaging-guided PCI. Additionally, 19 patients were excluded from the analysis due to registration errors related to the process of obtaining informed consent. Following this incident, we have implemented proactive measures to prevent similar occurrences in the future, and such errors have not recurred. Second, although the incidence of contrast-induced nephropathy after PCI was extremely low and comparable between the 2 groups, patients at high risk of contrast-induced nephropathy (i.e., severe renal dysfunction at baseline) were not included in the study. Third, the rate of recruitment in our study was low (5.2%), primarily because of the onset of the COVID-19 pandemic, which imposed significant restrictions on hospital admissions and patient care. Our patient enrolment period coincided with the initial phase of the pandemic, leading to challenges in recruiting eligible patients. Despite the protocol requiring rehospitalisation for follow-up OFDI, the restrictions imposed during the pandemic made it difficult to adhere to the study protocol and enrol patients as planned. Fourth, in our study, each clinical event was adjudicated by the respective institution rather than by an independent committee. Finally, the follow-up period was limited to 12 months. Further studies with longer follow-up periods are required to address the long-term impact of these imaging-guided procedures.
Conclusions
Detailed IVUS and OFDI analyses with a blinded comparison confirmed several differences in local findings between OFDI- and IVUS-guided PCI in patients with ACS. The MLA on 8-month follow-up OFDI was comparable between the 2 groups, suggesting that both OFDI- and IVUS-guided PCI are similarly feasible for coronary intervention using current-generation DES. Given the post-PCI findings, OFDI guidance may be a potential option for PCI in ACS.
Impact on daily practice
Among patients with acute coronary syndrome (ACS), optical frequency domain imaging (OFDI)- and intravascular ultrasound (IVUS)-guided percutaneous coronary intervention (PCI) were equally safe and feasible, with comparable in-stent minimum lumen areas at 8 months. IVUS-guided PCI may facilitate larger stent sizing than OFDI-guided PCI, whereas OFDI-guided PCI may reduce the risk of stent edge dissection and a final Thrombolysis in Myocardial Infarction flow of <3 following PCI. Our results indicate that given the post-PCI findings, OFDI guidance might be an option for PCI in ACS patients.
Funding
This work was supported by a grant from Terumo Corporation. However, the company was not involved in the design, data collection, data analysis, data interpretation, writing of the report, or decision to submit the article for publication. The corresponding author had full access to all data in the study and had the final responsibility for the decision to submit the manuscript for publication.
Conflict of interest statement
T. Akasaka is a medical advisor of Terumo Corporation (employee). The other authors have no conflicts of interest relevant to the contents of this paper to declare.Impact on daily practiceAmong patients with acute coronary syndrome (ACS), optical frequency domain imaging (OFDI)- and intravascular ultrasound (IVUS)-guided percutaneous coronary intervention (PCI) were equally safe and feasible, with comparable in-stent minimum lumen areas at 8 months. IVUS-guided PCI may facilitate larger stent sizing than OFDI-guided PCI, whereas OFDI-guided PCI may reduce the risk of stent edge dissection and a final Thrombolysis in Myocardial Infarction flow of <3 following PCI. Our results indicate that given the post-PCI findings, OFDI guidance might be an option for PCI in ACS patients.
Supplementary data
To read the full content of this article, please download the PDF.

