In this issue of EuroIntervention, two interesting image reports describe the OCT appearance of restenotic tissue of sirolimus-eluting stents and both refer to the “black hole” phenomenon that was first described as an observation in IVUS-studies of restenotic tissue of patients after radioactive stent implantation or brachytherapy1,2. In some of these patients echolucent restenotic tissue was detected with a remarkable homogeneous black appearance without backscatter and was termed “black hole”1. These lesions were clearly distinguishable from echoreflective neointimal hyperplasia1. Atherectomy samples from these lesions revealed a hypocellular matrix (probably at least partly due to cell death) rich in proteoglycan and poor in mature connective tissue elements, such as collagen-matrix and elastin1,3.
Later, a “black hole” phenomenon was also described in the restenotic tissue of some patients after sirolimus-eluting stent implantation4. In patients who had a sirolimus-eluting stent implanted after failure of previous intracoronary brachytherapy, black holes were rather frequently observed by IVUS analysis (approximately 20% of patients)5. In patients without a previous brachytherapy, the black hole phenomenon was observed more rarely. An analysis of IVUS exams in 102 consecutive patients with sirolimus-eluting stent restenosis reported a “black hole” in eight patients6. Recent reports from a histological analysis of atherectomy samples of restenotic tissue from sirolimus-eluting stents with “black hole” IVUS-appearance suggested that organised thrombotic material with fibrin can also cause such an echolucent image phenomenon after sirolimus-eluting stent implantation7,8. Recent studies have suggested that low OCT-signal intensity can be related to different tissue composition after DES implantation.
Low OCT/OFDI signal intensity due to proteoglycan-rich/collagen-matrix poor restenotic tissue or fibrin-rich thrombus formation
The two cases reported in the present issue of EuroIntervention are referring to the “black hole” phenomenon and are suggesting that the observed low OCT signal intensity in areas of restenotic tissue is related to cell death and low amounts of organised mature connective tissue elements, such as collagen matrix (although no histological analysis has been performed). The case reported by Kurita et al describes the OCT appearance of a transient “black hole” phenomenon by IVUS two months after sirolimus-eluting stent implantation9. OCT revealed a layered structure with an inner high signal intensity layer and outer layers of low OCT signal intensity. Of note, a previous report has described the histological examination (after atherectomy) of two sirolimus-eluting stent restenotic lesions with low OCT signal intensity and has suggested that this can be due to both, myxoid tissue or fibrin-rich thrombus formation10. However, myxoid tissue appeared as a layered structure by OCT whereas another case with fibrin-rich thrombus formation had a more irregular, patchy structural OCT appearance, supporting the possibility that the observation in the present case may represent myxoid tissue.
In the case reported by Linares et al, OCT findings of a patient who had restenosis two month after paclitaxel-coated balloon angioplasty of sirolimus-eluting stent restenosis are presented11, revealing large homogenous-appearing elliptic areas with low OCT signal-intensity. The shape of the lesions is strongly reminiscent of the “black hole” images using IVUS. As described in this editorial, several different restenotic tissues can appear with low OCT signal-intensity. However, the regular border and structure of the low OCT signal-intensity areas of this case raise the possibility that the restenotic tissue with OCT “black hole” appearance may result from cell death and low amounts of organised mature connective tissue elements.
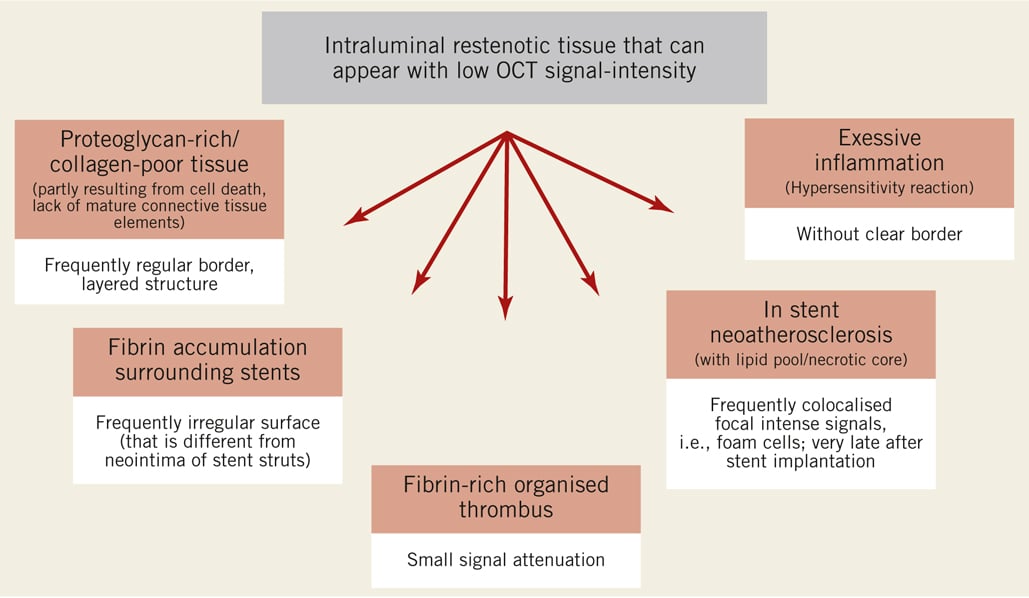
Figure 1. There are several intraluminal restenotic tissues which can appear with a low OCT signal intensity, including proteoglycan-rich/collagen-poor tissue (such as the “black hole” appearance after intracoronary radiation), fibrin accumulation, fibrin-rich organised thrombus, in-stent neoatherosclerosis and excessive inflammation. Therefore, the OCT signal intensity alone is not sufficient to distinguish between these different restenotic tissues. However, there are additional characteristics or associated signals of these different tissues that may aid in their distinction, some of which are noted in the figure.
Low OCT/OFDI signal intensity of fibrin accumulation surrounding stent struts
Of note, several recent studies have described that fibrin accumulation surrounding stent struts appears by OCT/OFDI with a lower signal intensity as compared to neointima without fibrin accumulation12,13. We have observed in a study at different time points after porcine coronary stent implantation, that fibrin-rich tissue (as detected early after stent implantation and confirmed by electron microscopy) had a substantially lower OCT signal-intensity when compared to neointima-covered stent struts later after implantation that were covered by smooth muscle cell-rich tissue with extracellular matrix containing collagens12. Another recent study has compared the OCT/OFDI and histological appearance of tissue surrounding stent struts from human stents at autopsy13. A clear difference in the OFDI signal intensity was observed between fibrin accumulation and normal neointima tissue, and signal attenuation was greater for fibrin13. Interestingly, we, as well as Nakano et al, have observed that the luminal surface appears mostly irregular by OCT when stent struts are covered by fibrin accumulation as opposed to neointima12,13, suggesting that additional structural features may help in the characterisation of tissue with low OCT signal-intensity.
Low OCT/OFDI signal intensity of in-stent neoatherosclerosis
In-stent neoatherosclerosis represents another possible cause of late restenotic tissue with low OCT signal intensity. OCT examination has recently supported the concept that neoatherosclerosis may contribute to late drug-eluting stent restenosis and may contribute to clinical events in these patients, such as by neointimal rupture14, representing another possible mechanism in addition to impaired stent healing/coverage resulting in very late stent thrombosis15. The frequency of neoatherosclerotic lesions reported by OCT was higher as compared to autopsy histological studies16. A potential overestimation of in-stent neoatherosclerosis by OCT can therefore not be excluded. For example, the distinction between fibrin accumulation and a lipid core may be challenging17, and more experience and validation studies will be needed.
In summary, high-resolution of OCT/OFDI can give us further interesting insights into the vascular response after stent implantation. The relation between OCT/OFDI characteristics, including signal intensity and attenuation, and the underlying tissue composition is an area requiring further investigation. Large OCT registries will be required to gain more insights into the OCT appearance of different types of in-stent tissues and their potential clinical implications.
Conflict of interest statement
U. Landmesser has received research grant support from Terumo and Orbus Neich.
References