Abstract
Aims: The study investigates the safety and efficacy of primary stenting of infrapopliteal lesions using the Chromis Deep stent (Invatec, Roncadelle, Italy) in patients presenting with critical limb ischaemia (CLI).
Methods and results: Fifty patients with infrapopliteal arterial occlusive disease were included in the study from March until November 2007. All patients presented with CLI, 42 (84.0%) and 8 (16.0%) were diagnosed as Rutherford class 4 and 5, respectively. Procedural success was achieved in all 50 patients. Fifty study lesions were treated with angioplasty using the Amphirion Deep balloon and followed by the insertion of 58 Chromis Deep stents. After intervention patients were followed at 1, 6 and 12 month intervals with clinical examination and arterial duplex study. The survival of the studied population was 79.8%, the limb salvage was 91.5% and primary patency of treated vessel was 52.9% at 12 months.
Conclusions: Primary stenting using the balloon-expandable Chromis Deep stent in combination with the Amphirion Deep balloon is a safe treatment strategy for infrapopliteal lesions in patients with CLI.
Introduction
Over the last few years, different reports have been published about the endovascular treatment of infrapopliteal lesion in patients presenting with critical limb ischaemia (CLI). To date, percutaneous transluminal angioplasty (PTA) is considered to be the endovascular treatment of choice, and stent implantation is generally restricted to those cases with suboptimal outcome after PTA1,2. As the diameter of infrapopliteal vessels is similar to those of coronary vessels, and as no dedicated infrapopliteal stents were available, most manuscripts describe the implantation of coronary stents in the infrapopliteal bed. The limited flexibility and lengths of coronary stents make them perfectly match with the coronary anatomy and the morphology of the to be treated coronary lesions, but might be suboptimal for the treatment of the mostly long-length, diffuse lesions in the infrapopliteal vascular bed. This might have impeded the infrapopliteal stenting results, as well as perhaps being the cause that up until now the indication remains limited to bail-out stenting, whereas in other vascular beds primary stenting has gained popularity.
With the introduction of the Chromis Deep stent (Invatec, Roncadelle, Italy), the first flexible, long-length, balloon-expandable stent specifically designed for the use in the infrapopliteal arteries recently became available. The primary objective of this prospective, single-arm study was to evaluate the safety and efficacy of primary stenting using the Amphirion Deep balloon in combination with the Chromis Deep stent (Invatec, Roncadelle, It.), in patients presenting with critical limb ischaemia (CLI).
Methods
This prospective, non-randomised clinical trial was conducted with institutional ethics committee approval at the AZ-St Blasius Hospital, Dendermonde and the Imelda Hospital, Bonheiden, Belgium. Between March and November 2007, 50 consecutive CLI-patients with diagnosed infrapopliteal artery lesions were included in the study if they complied with the inclusion and exclusion criteria as listed in Table 1.
Thirty-two male (64.0%) and 18 female (36.0%) patients were included in the study. The mean age of the total study population was 75 years (range 52-87 years). The medical history revealed arterial hypertension in 43 (86.0%), diabetes in 31 (62.0%) and hypercholesterolaemia in 21 (42.0%) patients. Twenty patients (40.0%) admitted the use of nicotine. Thirty-eight (76%) patients were previously treated for peripheral and/or coronary artery interventions.
All patients included in the study presented with CLI: 42 (84.0%) belonged to Rutherford class 4 and 8 (16.0%) were diagnosed as Rutherford class 5.
Device description
The Chromis DEEP is a premounted cobalt chromium (CoCr) balloon-expandable stent with a dedicated design for the infrapopliteal vascular bed. The stent has a high radial force at low strut thickness and is available in diameters from 2.0 up to 4.0 mm and lengths ranging from 10 to 76 mm. This wide range of sizes allows treatment of both short lesions at the trifurcation as well as even long dissections or diffusely stenosed arteries. The stent is mounted on a flexible low profile delivery system and is compatible 0.014” guidewires. The over-the-wire coaxial shaft delivery system provides high pushability and optimal tracking through distal vessels, which is often required to pass the lesions. Both shaft lengths of 120 and 150 cm were available, allowing both the ipsi- and contralateral access approach.
The Amphirion DEEP balloon dilatation catheter was available in diameters from 1.5 to 4.0 mm and lengths from 20 to 120 mm. It is an over-the-wire system and is 0.014” guidewire and 4 Fr introducer sheath compatible.
Procedure description
Vascular access was achieved via the contralateral groin and all inflow limiting lesions were treated according to the investigator’s standard clinical practice. Before lesion treatment, diagnostic angiography of the lesion area and distal run-off were performed. A 90 cm long 6 Fr sheath (Destination™, Boston Scientific, Maple Grove, MN, USA) was used and installed proximal of the target lesion to facilitate catheter advancement.
Under fluoroscopic control, an 0.014” guidewire was positioned intraluminally beyond the target lesion.
The Amphirion Deep dilatation catheter was advanced over the guidewire to the target lesion for predilation of the total lesion length. Balloon diameter and length were matched according the interventionalist’s visual estimation of the lesion characteristics during control angiography. A Dolphin™ disposable inflation device kit (Sedat, Irigny, France) containing a 1:1 mixture of radiographic contrast (Visipaque, GE Healthcare, Princeton, NJ, USA) and normal saline was attached to the balloon inflation port. Dilatation was followed by implantation of an appropriately sized Chromis DEEP stent (Invatec, Roncadelle, Italy). Post-dilatation was allowed at the discretion of physician. Immediate angiographic procedural success, defined as maximal 30% residual stenosis on visual assessment of the treated lesion. Haemostasis is acquired by manual compression or closure device.
Follow-up visits
Following intervention according to the standard care of CLI, patients are followed at 1, 6 and 12 months with clinical assessment, Rutherford categorisation and arterial duplex examination. Patients with clinical deterioration were referred for arteriography.
Endpoints
The primary endpoint was 12-month primary patency of the target lesion and was defined as the absence of:
A. Target lesion revascularisation (TLR), either endovascular or surgical.
B. Major amputation.
C. Untreated restenosis (>50%) or occlusion as determined by colour flow duplex ultrasonography (CFDU).
The secondary endpoints of the study were 12 months:
A. Limb salvage (defined as avoidance of a major amputation).
B. Survival of the patient.
Anticoagulation regime
Daily aspirin therapy was initiated and clopidogrel saturation (75 mg daily during at least four days, or in one loading dose of 300 mg the day prior to the procedure) was obtained prior to the procedure. Heparin was used during the procedure (bolus of 150 IU/kg body weight). The post-procedure antithrombotic regimen was that used according to the routine clinical practice of the hospital (75 mg clopidogrel, daily for at least one month – 100 mg aspirin, daily lifelong).
Statistics
The statistical analysis on the follow-up data of the entire patient group was done by the Kaplan-Meier estimation. All calculations were performed using the Med Calc statistical software version 9.2.0.1.
Results
The average procedural time was 46 minutes (20-70 min), average contrast dosage used was 87 ml (50-150 ml) and the mean fluoroscopy time was eight minutes (4-41 min).
The 50 infrapopliteal target lesions treated had a mean stenosis of 90.6% (80-100%). The mean lesion length was 52.2 mm (30.0-80.0 mm) and the reference diameter of the vessel was 3.1 mm (2.5-3.5 mm). Thirty-three lesions were calcified (66.0%) and two were ulcerative (4.0%). In total 11 (22.0%) lesions were reported to be occlusive and the remaining 39 (78.0%) were stenotic. The distribution of the target infrapopliteal lesions was as follows: 12 tibiofibular trunks (24.0%), 23 anterior tibial (46.0%), eight posterior tibial (16.0%) and seven fibular arteries (14.0%).
Twenty-nine (58.0%) lesions were located in the proximal and 21 (42.0%) in the distal half of the lower leg.
Treatment of inflow limiting lesions was required in 18 (36.0%) of patients. In all cases, the optimalisation of the inflow tract (two aorto-iliac and 16 femoropopliteal lesions) was successfully performed during the same intervention.
All lesions were predilated with an Amphirion Deep dilatation catheter with and average balloon diameter of 2.8 mm (2.5-3.5 mm) and length of 55.2 mm (30.0-80.0 mm). The average dilation time was recorded as 12.3 seconds (5.0-60.0 sec) and the applied pressure as 9.1 ATM (8.0-14.0 ATM). Suboptimal outcome after predilation was observed was in 15 (30.0%) patients, flow limiting dissection was reported in two (6.7%) and >50% residual stenosis in 13 (93.3%) cases.
The dilated lesions were all stented with in total 56 Chromis DEEP stents (stent/patient ratio of 1:12) (Figure 1).
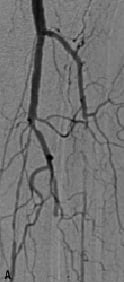
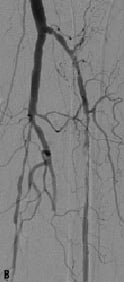
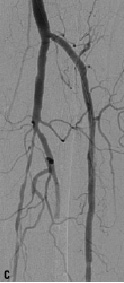
Figure 1. A 74 years old CLI patient (Rutherford 5) presenting with: a) long total occlusion of anterior tibial artery; b) suboptimal outcome after PTA with the Amphirion DEEP dilatation catheter (3.0x40.0) and; c) followed by Chromis DEEP stent (3.5x57.0) implantation.
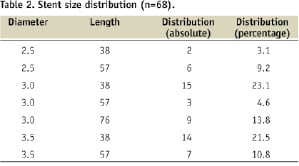
The distribution of the stent sizes used is given in Table 2.
The average stented length was 61.3 mm (38.0-95.0). Post-stent dilation was performed in two patients (5.0%) and post-procedural angiographic control revealed an average residual stenosis of 4.8% (0.0-20.0%). Immediate procedural success (below 30% residual stenosis) was achieved in all patients (100.0%). Haemostasis was achieved by manual compression in nine patients (18.0%) and a closure device in the remaining 41 patients (82.0%): the Angio-Seal STS PLUS (St. Jude Medical, St. Paul, MN, USA ) was used in 24 (58.5%) and the StarClose Vascular Closure System (Abbot Vascular, Redwood City, CA, USA) in 17 (41.5%) cases. There was no evidence of embolisation, thrombosis, perforation, or arterial spasm in any of the cases. There were no major access site complications or clinically relevant contrast nephropathy. The average hospitalisation time was 3.4 days (1-51 days). Forty-six patients (92.0%) were discharged within 24 hours after study intervention. Prolonged hospitalisation was required in four patients (8.0%) as these required in-hospital wound care.
Kaplan Meier estimation (Figure 2a-c) of the selected study endpoints, gave a result for the primary endpoint, a 12 month primary patency of the treated vessel of 52.9±7.4%.
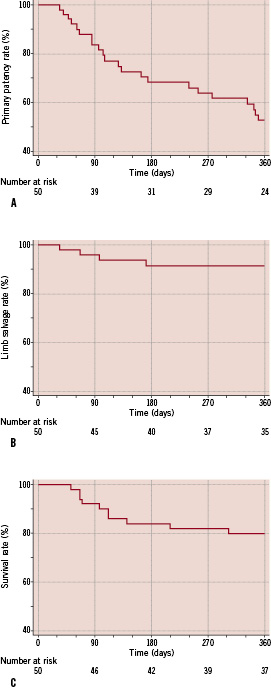
Figure 2. Kaplan-Meier estimation on follow-up results for total population. a) primary patency; b) limb salvage and; c) survival.
The rates for the 12 month limb salvage and survival, being the secondary study endpoints, were calculated by Kaplan Meier estimation and were 91.5±4.6% and 79.8±5.7%, respectively.
Subgroup analysis was performed to reveal predictive factors for stent failure. Stratification for lesions location (Figure 3a-b), revealed that patients presenting with lesions in the distal half of the lower leg had a significantly decreased primary patency compared to patients with proximal located lesions (hazard ratio 2.85; 95% CI 1.25 to 6.51). The 12-month primary patency rate for the proximal lesions was 68.9±9.2% and 33.3%±10.3% for the distal ones.
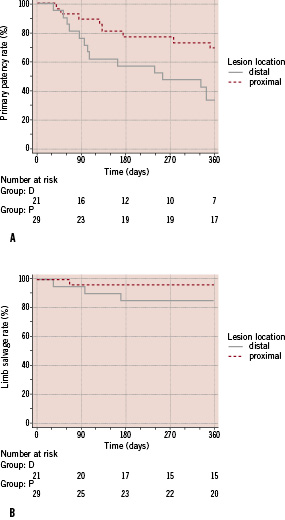
Figure 3. Kaplan-Meier estimation after stratification for lesion location. a) primary patency; b) limb salvage.
Log rank testing of the two curves revealed a significantly better rate for the patients with proximal lesions (p=0.0132). Concerning the limb salvage evolution in comparison to the lesion location, stratification did not reveal statistical different outcomes with log rank testing (p=0.1965) for the two subgroups. The 12-month limb salvage rate was 96.6±3.4% and 85.4±7.8% for the patients presenting with proximal versus distal lesions, respectively.
Comparison of primary patency curves after by log rank testing identified suboptimal outcome after predilation as predictive factor for patency loss (p=0.0025) (Figure 4a-b).
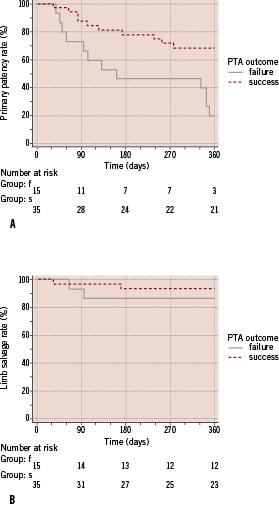
Figure 4. Kaplan-Meier estimation after stratification for outcome after predilation. a) primary patency; b) limb salvage.
Cases in which after predilation either a flow limiting dissection (n=2) or a residual stenosis (n=13) of at least 50% could be observed had, had over four times higher risk of patency loss (hazard ratio = 4.30; 95% CI 1,67 to 11,09). The 12-month primary patency was 20.0±10.3% for the patients with suboptimal outcome after predilation and was 68.4±8.3% for those with good angiographic result after predilation. No statistical difference was found for the limb salvage evolution for patients with or without suboptimal predilation outcome as found with log rank testing (p=0.4122). The 12-month limb salvage rate was 93.7±4.4% and 86.7±8.8% for the patients that had a successful predilation and suboptimal PTA outcome, respectively.
Detailed further analysis of the subgroup of patients that had a suboptimal predilation, demonstrated that in all cases in which a loss of primary patency within 12 months after index intervention was observed, residual stenosis ≥50% after predilation was noted as the cause. Flow limiting dissection after predilation did not result in patency loss within 12 months.
Considering the potential influence of inflow improving treatment on the outcome of Chromis DEEP, the comparison of the Kaplan Meier curves of the patients that required inflow optimisation with those with solitary by log rank testing did not reveal statistical differences for the either the primary patency (p=0.4512), or the limb salvage curves (p=0.6680).
Discussion
The Chromis DEEP stent is the first stent with a dedicated design to treat infrapopliteal lesions. The stent is designed to have an improved flexibility and is available in lengths up to 76 mm, which accommodates better the often diffuse aspect of these lesions.
Although, within this study, setting primary stent placement was performed, stenting of infrapopliteal lesions is normally reserved as bail-out procedure after suboptimal PTA. Still, different series of primary stenting (balloon expandable or self-expanding; bare metal, passive coated of drug eluting) for the infrapopliteal indication have been published3-8.
Feiring et al were the first to demonstrate the safety and utility of primary stenting using coronary stents in the tibial vessels3. The first RCT on the topic was published by the Vienna group, which concluded that the angiographic outcome after primary stenting, using a coronary balloon-expandable stent with a passive coating (InPeria Carbostent, Sorin, Biomedica, Italy), was superior to PTA alone in infrapopliteal vessels. Fifty-one patients presenting with CLI due to infrapopliteal lesions were randomised for treatment by PTA (53 lesions in 27 patients) or stent application (42 lesions in 24 patients). The median lesion length was 24.0 mm (5.0-30.0 cm). Considering a 50% restenosis threshold, the cumulative primary patency at six months was 79.7% for the stent and 45.6% for the PTA group (p<0.05). The limb salvage rate after six months was 97.4% after stent implantation4.
Another publication on primary stenting with bare metal balloon expandable coronary stents described the experience of our group using the Multilink Vision (Abbott Vascular, Redwood City, CA, USA) stent. In a cohort of 50 patients with CLI, 68 stents were implanted to treat 62 infragenicular lesions with a mean lesion length of 21.1 mm (5.0-52.0 mm). After 1-year, a limb salvage rate of 89.3% was calculated with a duplex derived primary patency of the treated vessel of 62.8%5.
Beside various types of bare-metal balloon-expandable coronary stents, also primary stenting with dedicated self-expanding nitinol stents shows to be a safe and effective strategy. The analysis of our series of 47 CLI patients treated with primary implantation of 67 Xpert nitinol stents (Abbott Vascular, Redwood City, CA, USA) in 58 infrapopliteal lesions (mean lesion length 32.4 mm [6.0-100.0 mm]), resulted in a 12 month primary patency and limb salvage rate of 76.3% and 95.9% respectively6.
Other authors investigated primary stenting using coronary balloon-expandable drug eluting stents. Commeau et al performed primary stenting using the Cypher sirolimus-eluting stent (Cordis, Miami, FL, USA) to treat 30 consecutive patients with CLI (Rutherford category 3-6) and a minimum of two diseased infrapopliteal vessels. Sixty-two arteries were treated with in total 106 Cypher stents, an average of 3.5 stents per patient. Direct stenting was performed in 20 patients (66%). A limb salvage rate of 100% was achieved at a mean follow-up of 7.7 months, and all surviving patients had marked clinical improvement with 97% primary patency as measured by TLR7.
Rosales et al performed primary stenting with both sirolimus and paclitaxel eluting stenting and published the outcome of a series of 24 patients with 28 target lesions. In total, 41 drug eluting stents (35 Cypher [Cordis, Miami, FL, USA] – 6 Taxus [Boston Scientific; Minneapolis, MN, USA]) were implanted leading to an average stent rate per lesion of 1.46. The average follow-up in their series was six months and they did present a primary patency rate of 89.0% with a limb salvage rate of 91.7%8.
Compared to the earlier published 12-month limb salvage rates, the 91.5% as recorded in our series, is compatable to the rates as found by other authors, while the 12-month primary patency rate of 52.9% is in the lower range. The rather low patency rate might be explained by the relative high number of lesions located in the distal half of the lower limb (42.0%) and the long length of the included lesions (52.2 mm) in comparison to the focal, and more proximally located, lesions as investigated in the previously published data sets.
Based on the results in our study population, we could identify different predisposing factors for early stent failure. The treatment of target lesions in the distal half of the lower leg and the presence of suboptimal outcome after predilation significantly decreased the durability of stent patency. Our findings on the influence of the lesion location are supported by our former publication on primary stenting with the nitinol Expert stent9. Considering the observation that suboptimal predilation, due to residual stenosis, limits the primary patency, indicates that even the stent could not optimally reconstruct the original anatomy due to plaque characteristics.
The question remains whether predilation should be performed in any infrapopliteal treatment, or whether direct stenting might be an option. As described by Commeau et al, direct stenting might avoid the risk for long dissection after predilation or multiple balloon inflations. Although there is still no evidence that exists to prove this hypothesis, they indicate that predilation should only be used for occlusions and/or the inability to cross the lesion with the stent catheter directly7.
The major limitation of this study is that it concerns a prospective non-randomised study which is limited to two centres and involving a small number of patients. The other limitation concerns the patency of the device which was determined by Duplex ultrasound examination, rather than conventional angiography. During this study inclusion period, Favaretto et al brought to light the fact that duplex ultrasound has only limited value for the investigation of infrapopliteal lesion10. Therefore any future investigation on the efficacy of the Chromis DEEP stent, or any other endovascular device for infrapopliteal revascularisation should be controlled by means of angiographic control rather than Duplex ultrasound examination.
Conclusion
The findings in the tested study population indicate that primary stenting with the Chromis DEEP stent is a safe technique for the management of CLI due to infrapopliteal lesions. The 12-month limb salvage rate was comparable to the published reports on other primary stenting studies for below the knee indications. The primary patency rate in this series seems to be at the lower range, which might be explained by the relative long length and distal location of the lesions treated in the study.
Acknowledgements
The authors take great pleasure in thanking the staff of the Flanders Medical Research Program (www.fmrp.be), with special regards to Koen De Meester, for performing the systematic review of the literature and providing substantial support for the data analysis and writing of this article.
Reference

