
Abstract
Aims: We aimed to evaluate the effect of omitting predilation on feasibility, procedural results and safety in balloon-expandable TAVI.
Methods and results: We performed an analysis of all 680 patients scheduled for a balloon-expandable TAVI prosthesis between January 2011 and August 2016. Patients treated with or without predilation were compared. Procedure times decreased from 85.6±42.9 to 56.7±26.1 minutes (p<0.001), fluoroscopy times from 9.5±5.7 to 6.2±3.9 minutes (p<0.001) and contrast volume from 131.9±60.8 to 85.4±37.4 ml (p<0.001) without predilation. Intraprocedural CPR was significantly more frequent in the predilation group (5.3% vs. 1.4%, p=0.01). Stroke rate was low at 1.5% and with no detectable difference. Applying VARC-2 definitions, the combined endpoints device success (88.3% vs. 92.4%, p=0.07) and clinical efficacy (88.7% vs. 92.4%, p=0.11) were comparable with or without prior valvuloplasty, while early safety was less frequent with predilation (85.2% vs. 90.2%, p=0.04). At 30 days, all-cause mortality and cardiovascular mortality were 6.8% with predilation vs. 2.9% without predilation (p=0.03) and 5.3% vs. 1.4% (p=0.01).
Conclusions: TAVI without prior valvuloplasty is feasible without apparent adverse impact in patients receiving a balloon-expandable TAVI prosthesis. The omission of predilation is associated with shorter procedure time, less radiation exposure and lower rates of intraprocedural resuscitation.
Abbreviations
CPR: cardiopulmonary resuscitation
CT: computed tomography
LVOT: left ventricular outflow tract
PVL: paravalvular leakage
RBC: red blood cells
TA: transapical
TAVI: transcatheter aortic valve implantation
TF: transfemoral
VARC-2: Valve Academic Research Consortium 2
VF: ventricular fibrillation
VSD: ventricular septal defect
Introduction
Since the introduction of transcatheter aortic valve implantation (TAVI) into routine clinical practice, balloon valvuloplasty of the stenotic aortic valve prior to the positioning of the TAVI prosthesis has been an integral part of the procedure first described by Cribier1,2. The rationale for predilation is to facilitate passage and full expansion of the TAVI prosthesis in analogy to vascular stent implantation. Balloon valvuloplasty requires a short period of circulatory arrest achieved by rapid ventricular pacing (>180 bpm) for up to 30 seconds. Potential complications such as prolonged hypotension, ventricular fibrillation and cardiopulmonary resuscitation have been described3-5. Inherent risks include thromboembolic events of aortic or valve debris, conduction disorders or annular rupture. Omission of predilation might therefore be beneficial besides simplifying the procedure. Single-centre case series have reported promising results of feasibility with self-expanding6-8 and balloon-expandable9,10 prostheses. Since 2013, an increasing number of TAVI procedures with balloon-expandable prostheses have been performed without predilation at our institution. Nonetheless, predilation is recommended in the manufacturers’ instructions, and a recent publication reported an increased number and extent of cerebral lesions when predilation was omitted11. This prompted us to review the routine practice in our centre. We analysed procedural characteristics, functional results and clinical outcome with respect to the performance of balloon valvuloplasty.
Methods
From 2011 all patients scheduled for TAVI were entered into a database. Predilation was first omitted in patients receiving a balloon-expandable valve via the transapical access. Subsequently, this strategy was also applied to patients treated via the transvascular access. Analysis included all consecutive patients who were scheduled for TAVI with a balloon-expandable valve. Patient selection was based on current guidelines and the indication for TAVI was confirmed by an interventional cardiologist and a cardiac surgeon. Baseline and procedural characteristics and the post-interventional course were documented in a standardised electronic documentation form. Laboratory results were imported electronically for classification of bleeding and acute kidney injury. The diagnostic workup included transthoracic echocardiography, coronary angiography and multislice computed tomography (CT) of the aorta, as previously described12. CT data of all patients were analysed to determine the dimensions of the aortic annulus using semi-automated software (3mensio Structural Heart™; Pie Medical Imaging BV, Maastricht, the Netherlands) introduced into our program in 2014. The degree of calcification of valve cusps, annulus and left ventricular outflow tract (LVOT) was judged qualitatively as none, mild, moderate or severe. A preset calcification window of >450 Hounsfield units was used.
All cases of suspected stroke were investigated by our onsite Neurology Department, including cerebral CT or magnetic resonance imaging, and confirmed or discarded. Before discharge, post-interventional mean gradient and the extent of regurgitation of the aortic prosthesis were determined by echocardiography. Survival status was determined by telephone call at 30 days and yearly thereafter. Analysis was performed according to the definitions of the Valve Academic Research Consortium-2 consensus document (VARC-2 criteria)13. Procedural characteristics and clinical outcomes were compared in patients with or without predilation. Analysis of patient data for scientific evaluation is legitimised by federal law and review of patient data was approved by our institutional ethics committee.
STATISTICAL ANALYSIS
Categorical variables are expressed as numbers and percentages. Continuous variables are expressed as means with standard deviation or as medians with quartiles if a non-normal distribution was assessed by the Kolmogorov-Smirnov test. For comparison of data between both groups, the chi-square test was used for categorical variables and the Mann-Whitney-Wilcoxon test for continuous variables. Where appropriate, Fisher’s exact test was used. All p-values were calculated by two-tailed tests. Statistical significance was defined at p<0.05. Analysis was performed on an intention-to-treat basis. Statistical analysis was performed with SPSS for Windows statistical software, Version 23 (IBM Corp, Armonk, NY, USA).
Results
Between January 2011 and August 2016, 680 patients were scheduled for balloon-expandable TAVI because of native aortic valve stenosis. Three hundred and seventy-three (373) patients were assigned to transfemoral (TF) and 305 to transapical (TA) access. Two were treated via transaortic access. During enrolment, the proportion of transfemoral implantations increased from 46% in 2011 to 74% in 2016, parallel to a routine omission of predilation from 2014 onwards (Figure 1). In TF TAVI, predilation was performed in 213 cases while in 160 cases predilation was omitted. For TA TAVI, 187 patients were treated with predilation and 118 without. Thus, the predilation group included 400 patients and the patient group without predilation consisted of 278 patients.
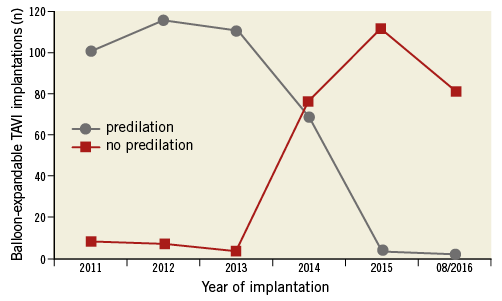
Figure 1. Development of balloon-expandable TAVI implantations from 2011 to August 2016 with and without predilation.
Baseline characteristics are summarised in Table 1. Patient groups with or without predilation were comparable irrespective of whether TA or TF TAVI was performed. The patient group without predilation had a higher proportion of male patients, mean left ventricular ejection fraction was slightly lower and arterial hypertension was less prevalent. While in both groups the majority of patients had TAVI for aortic stenosis, the number of patients with moderate or severe aortic valve regurgitation was higher in patients with predilation. Aortic valve area was slightly lower and mean gradients higher in the predilation group, while pulmonary artery pressures were identical. Accordingly, annular dimensions were smaller in the predilation group, congruent with a higher percentage of female patients. The degree of valve calcification decreased over time with a higher degree in the predilation group. In patients without predilation, 31% were still within the most severely calcified category.
The predominantly implanted prosthesis type was the SAPIEN XT in the predilation group (86%) and the SAPIEN S3 (95%) in the group without prior valvuloplasty (both Edwards Lifesciences, Irvine, CA, USA).
Procedural characteristics are shown in Table 2. The procedure was performed without predilation in 41% of all cases. Deployment of a single prosthesis within the intended position was feasible in 98% of all patients with or without predilation.
Median procedure time was 65 minutes (IQR 31-99). Mean procedure time decreased by 34% without predilation. In TF TAVI the mean duration of the procedure was 79±42 minutes with and 48±23 minutes without predilation (p<0.001), and in TA TAVI the respective values were 93±42 minutes with and 67±25 minutes without predilation (p<0.001). Fluoroscopy time decreased by 35%, area dosage by 42%, and use of contrast medium by 36%. Median contrast usage was 100 ml (IQR 40-160). Omitting predilation did not increase the rate of post-dilation. Post-dilation was performed in 25% in the predilation group and in 17% of cases without (p=0.02).
After TAVI, mean pressure gradients were higher in patients without predilation, but the mean absolute difference was small. Prosthesis dysfunction (e.g., aortic regurgitation/paravalvular leakage ≥grade 2 and mean gradient >20 mmHg) was observed in 10 patients (2.5%) in the predilation group and in nine patients (3.2%) without (p=0.57).
Adverse events and the composite endpoints device success and early safety are summarised in Table 3. Positioning of the prosthesis in the aortic annulus was feasible in all 278 patients without predilation. Conversion rates to alternative access, open heart surgery or extracorporeal bypass did not differ between groups irrespective of access route (Table 2). Cases of annular rupture or post-interventional VSD were anecdotal. Immediate procedural mortality (e.g., mortality within 72 hours after the procedure) occurred in six patients with a similar frequency (1%) in all subgroups.
Necessity of valve-in-valve implantation occurred in 11 cases and only affected the predilation group (p=0.005). It was more frequent with TA than with TF (5% vs. 1%, p=0.02).
Intraprocedural CPR was significantly more frequent in the predilation group (5.5% vs. 1.4%, p=0.01), while the occurrence of ventricular fibrillation (VF) (6.8% vs 3.2%, p=0.05) did not reach statistical significance.
The composite endpoint device success, defined as the absence of procedural mortality and a single prosthesis without valve dysfunction, was reached in 88% of all patients with and 92% of those without predilation (p=0.07). The main reasons for classification as device failure were mean gradients above 20 mmHg in small valve sizes and female patients.
In-hospital mortality was 5.7% without significant differences between groups. At 30 days, all-cause mortality and cardiovascular mortality in the predilation and no predilation groups were 6.8% vs. 2.9% (p=0.03) and 5.3% vs. 1.4% (p=0.01).
The 30-day stroke rate did not differ and was 1.8% with and 1.1% without predilation. Cerebral protection devices were applied in three cases. One stroke occurred after dislodgement of the protection device by the TAVI delivery system. Pacemaker implantation rates were 17% and 13%, respectively (p=0.19). In the TA subgroup, there was a higher rate with predilation (25% vs. 15%, p=0.04).
There was no difference in acute kidney injury stage 1 or 2 between groups. The difference in acute kidney injury stage 3 of 2.7% did not reach statistical significance. In two cases, new-onset chronic haemodialysis was initiated post-interventionally. Perioperative dialysis was significantly and chronic haemodialysis insignificantly more frequent in predilated patients.
The frequency of vascular surgery was 3.8% in TF TAVI and 2.3% in TA TAVI. Access-site complications were minor in 1.9% and major in 6.5%. Life-threatening bleeding occurred in 3.5%, major bleedings in 2.9% and minor bleeding in 6.9%. Transfusion of red blood cells (RBC) was performed in 14.5% of patients with TF and 39.7% with TA. At 30 days, the composite of all-cause mortality, stroke, life-threatening bleeding, acute kidney injury stage 2 or 3, coronary artery obstruction requiring intervention, major vascular complication or a repeat procedure for valve-related dysfunction (early safety) occurred in 59 patients (14.8%) with predilation and in 25 patients (9.0%) without (p=0.04).
A change of practice regarding predilation occurred in 2014, as sensitivity analysis of patient data for 2014 yielded virtually identical results with respect to patient characteristics, severity of aortic stenosis, valve dimensions and degree of calcification. Of 145 balloon-expandable TAVI, 48% were performed with prior valvuloplasty irrespective of access route. The TF group consisted of 87 and the TA group of 58 patients. Implanted valve type was the SAPIEN XT in 28 and the SAPIEN S3 in 117 cases. In TA and the overall cohort, significantly more S3 valves were implanted irrespective of the performance of predilation.
In this smaller sample, the main findings of procedure characteristics are confirmed. Procedure time decreased by 26% in TF and 27% in TA without predilation. Fluoroscopy time was reduced by 21% and area dosage by 45%. An average of 44 ml less contrast agent was used per patient. Results in TA and TF subgroups were congruent. While implanted valve sizes were slightly smaller in the TA group, this was not the case in TF and the pooled analysis. Post-dilation was more frequent in predilated patients in TA and the overall cohort of 2014. Due to the smaller sample size, the remaining parameters including intraprocedural VF and intraprocedural CPR did not reach statistical significance.
The analysis of adverse events and composite endpoints in 2014 could not demonstrate a statistical difference in the overall cohort or TA and TF subgroups due to the low event rate.
Discussion
Balloon aortic valvuloplasty was a manufacturer recommendation right from the introduction of balloon-expandable prostheses and all CE-mark trials were performed with predilation. Its performance allowed final assessment of the effectiveness of rapid pacing in reducing transaortic flow and served as an adjunctive sizing tool. Intuitively, preparation of the stenotic valve should facilitate crossing of the valve with large diameters, particularly in first-generation devices with slightly larger crimped profiles. Nevertheless, small series have reported the feasibility of direct valve implantation for SAPIEN XT as well as for SAPIEN 3 valves8-10. While the rationale for prior valvuloplasty in balloon-expandable TAVI appears plausible, we began to omit the predilation step in an attempt to simplify the procedure and reduce the number of rapid pacing episodes. Our predefined bail-out strategy was to withdraw the valve delivery system into the descending aorta and perform the predilation step via the contralateral femoral artery by retrograde passage of the stenotic valve with a second wire. Until now this bail-out manoeuvre has not had to be put into practice. Concerns that crossing the often heavily calcified stenotic valve without predilation could be difficult or inflict an increased stroke risk upon patients did not become reality in our treatment cohort. Other concerns were possible sequelae of the procedure itself on functional results and procedural safety.
Procedural parameters document the benefits, with reduction of about a third of procedure and fluoroscopy times with subsequent reduced radiation exposure and contrast medium usage. Of particular importance is that the need for intraprocedural resuscitation and the occurrence of ventricular fibrillation are halved. A relation to frequency and duration of rapid pacing episodes required for valve dilation with consecutive prolonged haemodynamic recovery is plausible. Other causes for resuscitation were infrequent. A 30-day mortality rate below 3% underlines the safety of omitting predilation. It is obvious that omitting predilation has an effect on at least procedural characteristics, exceeding the effect of operator experience.
There were differences in the sex distribution of our treatment groups, with a female predominance in predilated patients and a male predominance in patients with direct valve implantation, particularly in the TF subgroup. Correspondingly, the size of the implanted prostheses was significantly larger and EF slightly lower in the latter group. However, without predilation, transvalvular gradients were only marginally higher and the incidence of aortic insufficiency >I° did not differ between the groups. Post-dilation was more often performed in the predilation group dominated by patients treated earlier and thus receiving the SAPIEN XT valve more frequently. The higher post-dilation rate is possibly due to the issue of paravalvular leakage (PVL). The evolution of the Edwards SAPIEN S3 valve, which was used in more recent cases, may also have had an impact on PVL. However, there was certainly no apparent negative effect abstaining from prior valvuloplasty regarding post-ballooning and PVL. The same holds true for device success, bleeding, access-site complications, early safety and clinical efficacy. Of note is that there was no significant difference in the post-procedural incidence of third-degree AV block and an identical pacemaker rate in the TF subgroup and the whole cohort. The TA subgroup had a higher pacemaker rate with predilation.
A trend towards a lower incidence of acute kidney injury stage 3 was observed in patients without predilation. Lower contrast usage and fewer repetitive rapid pacing episodes with concomitant hypotension are possible explanations. It is of note that chronic kidney disease stage 3 or higher was present in >50% in our cohort, while large national registries report values of about 30%. The prognostic impact of renal impairment on survival after TAVI is increasingly recognised14. Postoperative haemodialysis was performed in 4% of registry patients, which is comparable to the 6% observed in our patient cohort.
The higher rates for intraprocedural CPR, valve-in-valve implantation and a trend towards more frequent acute kidney injury stage 3 are noteworthy but do not prove a causal relationship in our non-randomised exploratory analysis.
At 1.5% the stroke rate in our study population is lower than reported values in large studies, while intra-hospital mortality is identical at below 6%. This was not affected by the omission of predilation.
Limitations
The retrospective nature of our study implies that omission of predilation is only one of several aspects modified during the study period. Four aspects related to change over time pose relevant limitations to our analysis: learning curve, a gradual shift towards less calcified stenosis, device evolution and technological progress in CT quality and analysis. None of these issues can easily be quantified.
The predilation group represents largely early cases while the no predilation group consists mainly of more recent cases. Large registries show a parallel decrease in mortality over time15. The growing experience of the therapeutic team and a reduced degree of valve calcification as a surrogate for a shift towards a lower-risk population might have a similar effect on complication rates.
Another important change in 2014 was the replacement of the SAPIEN XT prosthesis by the next-generation balloon-expandable prosthesis SAPIEN S3. Plausible effects predominantly affect direct valve performance such as paravalvular sealing or prosthetic gradients. The most striking feature of the new valve was the sealing skirt, while valve preparation and the procedure itself remained largely unchanged. While direct valve implantation has been demonstrated to be feasible for the SAPIEN XT valve, we did not experience problems with valve crossing or deployment with the SAPIEN S3 valve.
Improvements in CT annulus and vascular sizing such as the introduction of semi-automatic software-aided measurement (3mensio Valve) in 2014 as well as improved CT raw data made it the unrivalled tool for procedure planning, valve sizing and device selection. It did not affect the aspect of predilation as it was abandoned generally after a very short transitional period.
A sensitivity analysis including only patient data obtained in 2014 (thereby minimising the bias introduced by experience and CT sizing) showed similar results when compared to the whole study cohort. It is obvious that no measure could adequately control for the change in practice during the study period. It should therefore be noted that the omission was an important aspect, but only one out of several measures resulting in a faster and simplified TAVI procedure.
However, the absence of an adverse effect allows the reverse conclusion. Despite all the limitations, our analysis revealed no apparent negative impact. Simplification of the procedure was associated with less radiation and fewer resuscitation episodes. The formal proof of a causal relation would require an adequately powered randomised trial which for several reasons is unlikely to be performed (cost in relation to possible benefit, reluctance to perform valvuloplasty in proponents of omission). Therefore, our study represents the best available evidence on this topic.
Conclusions
Omission of predilation in balloon-expandable TAVI does not have a negative impact. Radiation and contrast exposure were lower without superior functional results, higher stroke rate or better safety profile.
| Impact on daily practice Omission of predilation simplifies and shortens a complex procedure giving less opportunity for complications. Radiation exposure is significantly reduced for patient and interventionalist without compromising procedure results or patient safety. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

