Abstract
Sleep apnoea occurs in 5% to 10% of the general population1. Obstructive sleep apnoea (OSA) is the most common disease associated with resistant hypertension. In a paper published by Pedrosa et al, OSA - defined as an apnoea-hypopnoea index (AHI) >15 events/hour measured in polysomnography –was diagnosed in 64% of patients with resistant hypertension2. OSA is also considered as an independent risk factor for cardiovascular events: ischaemic heart disease, heart failure, stroke and death3. Several mechanisms, including oxidative stress, inflammation and endothelial dysfunction may be responsible for the association between OSA and cardiovascular disease4,5. Continuous positive airway pressure (CPAP) is a treatment of choice to reverse severe OSA and its consequences6,7.
Epidemiology and pathophysiology of obstructive sleep apnoea
In the general population and in patients with cardiovascular diseases, OSA is two to three times more common in men than in women, and in the elderly rather than in the young6. OSA is an independent risk factor for cardiovascular events, including ischaemic heart disease, stroke, heart failure and death. Obesity is also associated with OSA in patients with hypertension3,8, while a direct relationship between body mass index and OSA severity in patients with heart failure and stroke has not been clearly established9-11.
OSA is characterised by recurrent episodes of partial or complete upper airway (UA) obstruction during sleep12. Apnoea arises when sleep-related inhibition of the respiratory drive to the UA dilator muscles is superimposed on a previously narrowed airway6. The UA may be narrowed due to tonsillar hypertrophy, macroglossia or if the UA is surrounded by soft tissue mass containing fat deposits which is increased in obese patients with large neck circumferences. Also, peripharyngeal fluid retention, and its rostral nocturnal shift while sleeping, may increase peripharyngeal tissue mass. In different clinical scenarios, amplified sodium and water retention may be: dietary13; neurogenic, resulting from increased sympathetic activity leading to augmented renin release and sodium retention14; or humoral, as a consequence of activation of the renin-angiotensin-aldosterone axis15. It has been demonstrated that, in response to the application of lower body positive pressure, neck circumference increases and the pharyngeal cross-sectional area decreases resulting in higher pharyngeal resistance and collapsibility16,17. Redolfi et al reported a direct relationship between the volume of the nocturnal fluid shift and the change of neck circumference and severity of OSA assessed by AHI18. Other evidence that nocturnal rostral fluid shift can cause OSA was provided by two studies demonstrating that fluid removal using overnight peritoneal dialysis in patients with chronic renal failure increased pharyngeal UA diameter and alleviated OSA severity as compared to the removal of the same amount of fluid with continuous 24-hour dialysis19-21.
OSA and hyperaldosteronism are very common in subjects with resistant hypertension and aldosterone-mediated chronic fluid retention may influence OSA severity in patients with resistant hypertension. In an observational study by Gaddam et al, treatment with a mineralocorticoid receptor antagonist substantially reduced the severity of OSA15. In conclusion, these studies provide evidence favouring the concept that nocturnal fluid shift contributes to the pathogenesis of OSA, and fluid volume may determine OSA severity.
Sympathetic neural mechanisms in obstructive sleep apnoea and resistant hypertension
Increased sympathetic activity, consistently evident in OSA patients, likely plays a key role in the development of resistant hypertension. Autonomic and haemodynamic responses to obstructive sleep apnoea are complex and include the effects of apnoea, hypoxia, hypercapnia, the Mueller manoeuvre (inspiration against a closed glottis) and arousal22. Hypoxia and hypercapnia act synergistically to increase sympathetic activity and this increase is especially marked during apnoea23-26. The role of sympathetic activation in OSA patients was further elucidated in a study by Somers et al27. Patients with OSA had high sympathetic activity when awake, with further increases in blood pressure and sympathetic activity during sleep. These increases were attenuated by treatment with CPAP indicating that OSA induces sympathetic activation and blood pressure rises during sleep. Sympathetic overactivation in OSA patients also acts to increase heart rate28, and can worsen the prognosis of patients with cardiovascular diseases specifically by causing cardiac ß-adrenoreceptor desensitisation, arrhythmias, myocyte injury and necrosis, peripheral vasoconstriction, and promoting renal sodium retention, both directly and through stimulation of the renin-angiotensin-aldosterone axis29.
Relationship between obstructive sleep apnoea and hypertension
The prevalence of OSA in primary hypertension is ≈35%30 and in patients with drug-resistant hypertension over 60%2,3. It is known that intermittent hypoxia or experimentally induced OSA can cause persistent daytime hypertension in rats and dogs31,32. It was observed that subjects with an AHI ≥15 had almost a three-fold greater likelihood of developing hypertension than those with an AHI of 033; however, other studies did not confirm this association34,35. Nevertheless, the remarkable finding that OSA is by far the most common disease associated with drug-resistant hypertension –and that its treatment may lower blood pressure– suggests that OSA plays a provocative role in hypertension.
Renal denervation in resistant hypertension and obstructive sleep apnoea: animal studies
In an experimental study by Linz et al, renal denervation (RDN), but not the administration of ß-blockers, inhibited postapnoeic blood pressure rises in pigs36. In another paper, RDN induced elevation of urine volume and sodium excretion in rats with acute total obstructive apnoea37. This suggests that the increase of the renal sympathetic nerve activity during apnoea episodes does prevent the elevation of renal excretory function and further supports the hypothesis that renal sympathetic nerves play an important role in sodium homeostasis, with renal nerve activation enhancing sodium retention. Suppressing the sympathetic activity by RDN may impose the opposite effect.
Renal denervation in resistant hypertension and obstructive sleep apnoea: the human experience
The potential impact of RDN on the course of sleep apnoea was reported in one human study38. The study included 10 patients with resistant hypertension (defined as a systolic office blood pressure of greater than 160 mmHg despite treatment with three or more antihypertensive drugs, including diuretics) and sleep apnoea. OSA was diagnosed in eight patients, and mixed sleep apnoea (obstructive and central) in two. There were five patients with mild sleep apnoea (AHI five to 15 events/hour) and five patients with moderate-to-severe apnoea (AHI >15 events/hour). Two patients were treated with CPAP before RDN, and this therapy was maintained during the follow-up period. All patients underwent catheter-based radiofrequency RDN (Symplicity; Medtronic, Minneapolis, MN, USA). The median reduction of systolic blood pressure was 22 mmHg (p<0.01) and 34 mmHg (p<0.01) at three and six months after RDN, respectively (Figure 1), with no differences between the patients with mild and moderate-to-severe sleep apnoea.
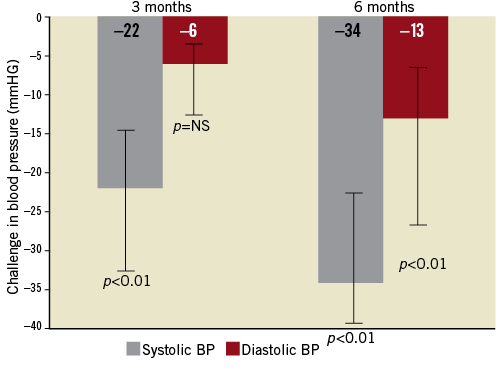
Figure 1. Median systolic and diastolic BP changes after a renal sympathetic denervation procedure at three and at six months of follow-up. Error bars represent interquartile range.
Decreases in AHI at three months (non-significant) and at six months (with a tendency towards significance) after RDN were noted (median 16.3 events/hour before RDN versus median 4.5 events/hour; p=0.059). Also, decreases in oxygen desaturation indices (ODI) at six months (median 13.0 events/hour before RDN versus median 8.7 events/hour; p=0.11) and decrease in median Epworth Sleepiness Scale score at six months (9.00 points versus 7.00 points; p<0.05) were observed. In summary, in eight out of 10 patients, an improvement in AHI was observed at six months (Figure 2). There were two patients with mixed sleep apnoea. In one of them, a reduction in sleep apnoea indices was also observed with a change in AHI –30.5 events per hour at six months. In patients with improvements in AHI, a significant decrease in 24-hour, daytime and night-time ABPM levels was observed, the latter being most pronounced (median: –8/–4 mmHg, –12/-5 mmHg and –10/–8 mmHg for 24-hour, daytime, and night-time, respectively; p<0.05 for all).
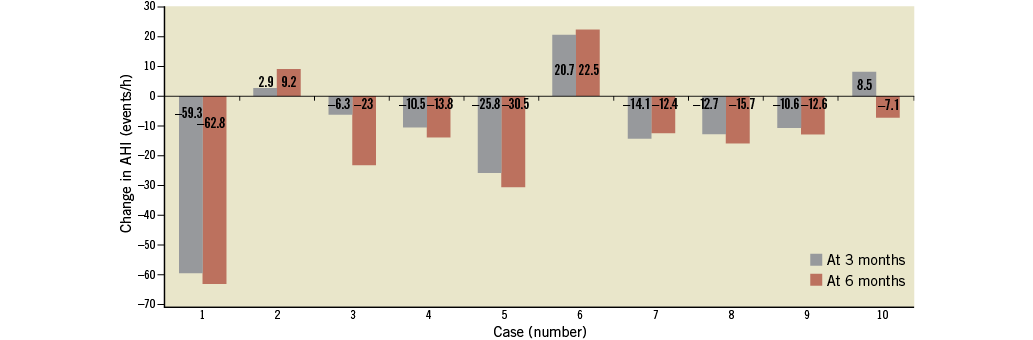
Figure 2. Changes of AHI at three and six months after denervation. Data of individual cases.
Along with blood pressure reduction and an improvement in the course of sleep apnoea, significant decreases in plasma glucose concentration two hours after glucose administration at three and at six months (median 7.0 mmol/dL vs. median 6.4, mmol/dL at six months; p<0.05) and in haemoglobin A1C level at six months (median 6.1% versus median 5.6%; p<0.05) were observed.
This study confirms that RDN lowers blood pressure in patients with resistant hypertension39,40 and endorses the work of Mahfoud et al documenting that RDN in humans improves indices of insulin action and glucose metabolism41. As well, this publication extends previous work by documenting that the blood pressure and metabolic benefits of renal denervation include patients with sleep apnoea and improve the course of the disease. Because these data are observational, the study cannot identify the exact mechanism responsible for any amelioration of sleep apnoea. Nonetheless, it should be emphasised that RDN influences key mechanisms regulating sympathetic activation. The efferent sympathetic renal nerves can affect control of renal vascular resistance, increase renin release and regulate sodium and water excretion13. The afferent renal nerves enhance the activity of the sympathetic nervous system. It has been also suggested that, in conditions of high-sodium dietary intake, activation of the afferent renal nerves contributes to the arterial baroreceptor-mediated suppression of efferent sympathetic renal nerves in the overall goal of preventing sodium retention and maintaining water and sodium homeostasis13,42. Therefore, RDN in patients with resistant hypertension and OSA might attenuate the effects of sympathoactivation additionally and independently of CPAP treatment. Lastly, it needs to be considered whether the fall in blood pressure may itself contribute to the attenuation of sleep apnoea.
Summary
Obstructive sleep apnoea is an independent risk factor for cardiovascular events, including ischaemic heart disease, stroke, heart failure and death. Obstructive sleep apnoea is also the most common disease associated with resistant hypertension. As both hypoxia and hypercapnia result in increased sympathetic activity, the sympathetic nervous system plays a key role in the development of resistant hypertension in patients with obstructive sleep apnoea. Sympathetic overactivation in OSA patients can worsen the prognosis of those patients with cardiovascular diseases, specifically by causing arrhythmias, myocyte injury and necrosis, peripheral vasoconstriction and the promotion of renal sodium retention, both directly and through stimulation of the renin-angiotensin-aldosterone axis. Preliminary results show that catheter-based renal sympathetic denervation may not only lower systolic blood pressure by ≈30mmHg in resistant hypertensive patients with sleep disordered breathing, but also improve sleep apnoea severity. Along with blood pressure reduction and improvement in the course of sleep apnoea, significant decreases in plasma glucose concentration in haemoglobin A1C levels two hours after glucose administration were observed.
Renal sympathetic denervation may conceivably be a potentially useful therapeutic option for this subset of patients; however, large randomised controlled clinical trials are needed to confirm the initial proof-of-concept data.
Conflict of interest statement
A. Witkowski has received a research grant and consultancy fees from Medtronic. J. Kądziela has received consultancy and proctoring fees from Medtronic.

