
Abstract
Aims: The aim of this study was to investigate whether a Heart Team decision-making process regarding the choice of revascularisation strategy based on non-invasive coronary multislice computed tomography angiography (MSCT) assessment of coronary artery disease (CAD) is equivalent to the standard-of-care invasive angiography-based assessment in patients with multivessel CAD.
Methods and results: The SYNTAX III Revolution trial is a prospective, multicentre, all-comers randomised trial that will randomise two Heart Teams to select between surgical and percutaneous treatment according to either an invasive conventional angiography or a non-invasive MSCT angiography assessment in patients with multivessel CAD. The treatment selection by each Heart Team will be guided by the SYNTAX score II calculation. The primary endpoint is the level of agreement according to kappa of the initial decision by the Heart Teams on the modality of the revascularisation based on MSCT and angiography assessments. Secondary endpoints include agreement on the number of vessels requiring treatment and the coronary segments in need of revascularisation.
Conclusions: The SYNTAX III Revolution trial will provide valuable information regarding the ability of a purely non-invasive coronary anatomy assessment to select accurately the most appropriate revascularisation strategy for patients with multivessel CAD.
Introduction
For the last 50 years, revascularisation along with optimal medical therapy has been the cornerstone of coronary artery disease (CAD) treatment in a large number of patients1. Since the introduction of coronary artery bypass surgery (CABG) in 1964 and percutaneous coronary intervention (PCI) in 1977, revascularisation procedures have undergone remarkable technological improvements that have increased their safety and efficacy, leading to their widespread application all over the world2,3.
Invasive coronary angiography has been the gold standard diagnostic method to guide revascularisation procedures since their introduction. As its limitations in some situations have been recognised, intravascular imaging and intracoronary pressure measurements were introduced to help overcome some of these limitations4,5. Nevertheless, one of the major remaining drawbacks of conventional coronary angiography is its invasive nature.
MULTISLICE CORONARY COMPUTED TOMOGRAPHY ANGIOGRAPHY (MSCT)
In the last two decades, coronary MSCT has been introduced as a non-invasive alternative for coronary anatomy assessment and has experienced considerable advances in the diagnosis of CAD6. Its diagnostic accuracy as compared to conventional invasive angiography has been tested in multiple studies7,8.
These data suggest that non-invasive coronary MSCT imaging is a good alternative for the assessment of coronary artery disease. Its non-invasive nature, along with its ability to evaluate coronary plaques, makes it an attractive diagnostic tool for the diagnosis and management of CAD patients.
ANATOMIC SYNTAX SCORE AND THE “HEART TEAM” CONCEPT
The anatomic SYNTAX score (www.syntaxscore.com) is a tool developed in the landmark SYNTAX trial to help cardiologists and surgeons in assessing the coronary angiogram and deciding when equivalent revascularisation can be achieved with CABG and PCI9-16. It classifies multivessel CAD patients into low, moderate or high-risk categories for major adverse cardiovascular events (MACE) based on the anatomic complexity for PCI.
After the publication of the SYNTAX trial, the score was shown to have important prognostic value as well as good ability to guide the decision-making process regarding the choice of revascularisation strategy17-19. The most recent European and American guidelines on coronary revascularisation recommend the use of the anatomic SYNTAX score to help cardiologists in selecting the most appropriate revascularisation modality in patients with multivessel CAD1,20,21.
FUNCTIONAL SYNTAX SCORE
Percutaneous coronary intervention guided by the assessment of the functional significance of a lesion using fractional flow reserve (FFR) has been shown to reduce inappropriate revascularisation whilst simultaneously improving clinical outcomes22,23. The functional SYNTAX score uses the principle of the functional assessment of coronary lesions to determine the SYNTAX score, rather than the angiographic determination of the SYNTAX score based upon visual assessment, as is undertaken in conventional anatomical SYNTAX score calculations24.
CT SYNTAX SCORE AND CT FUNCTIONAL SYNTAX SCORE
The anatomic SYNTAX score has been applied to non-invasive coronary MSCT images with good comparability. In an analysis by Papadopoulou et al, good correlation was found between invasive angiography SYNTAX score and coronary MSCT SYNTAX score (r=0.76, p<0.001)25. This study also showed good reproducibility with the kappa value for intra-observer variability showing substantial agreement (k=0.80)25.
The CT SYNTAX score can incorporate the FFRCT data (HeartFlow® Analysis; HeartFlow Inc., Redwood City, CA, USA) and generate the CT functional SYNTAX score. This new score will be validated in the CT substudy of the ongoing SYNTAX II trial.
SYNTAX SCORE II
Despite the good performance of the anatomic SYNTAX score in discriminating risk categories regarding outcomes, it is known that the coronary anatomy is not the only factor driving prognosis in coronary disease. Several clinical comorbidities present in patients with CAD can influence procedural results for both PCI and CABG. Furthermore, the comorbidities that negatively influence PCI might be different from the ones that have a negative impact in CABG results.
For that reason, within SYNTAX score-derived categories there can be patients with different risk profiles associated with the two modalities of revascularisation26. Thus, the need for a risk assessment tool that could provide individual assessment of the risk associated with both revascularisation strategies was evident. The SYNTAX score II was designed to fulfil this need27. This new score was created by combining the anatomic SYNTAX score with clinical variables that were shown to interact with either or both revascularisation modalities and/or to have an impact on long-term mortality. These clinical variables included age, creatinine clearance, left ventricle ejection fraction, presence of unprotected left main disease, gender, chronic obstructive pulmonary disease and peripheral vascular disease28,29.
The score generates a prediction of four-year mortality for both modalities and provides a treatment recommendation based on these predictions. When the two predicted mortality rates (for CABG and PCI) cannot be separated with 95% confidence, the score gives an equipoise recommendation (treatment with either modality). When the predicted mortality is significantly lower with one of the two strategies, that strategy is recommended. This new score is recommended in the most recent European revascularisation guidelines as a tool to guide the decision-making process regarding the choice of the revascularisation strategy in patients with multivessel CAD1. It also forms the basis of the design of the ongoing SYNTAX II trial (ClinicalTrials.gov number, NCT02015832) that is enrolling patients to undergo current state-of-the-art PCI guided by FFR and intravascular ultrasound30. Patients are included when the score gives a lower predicted PCI mortality as compared to CABG or when there is equipoise in mortality predictions between the two strategies.
MSCT SYNTAX SCORE II
In the MSCT substudy of the ongoing SYNTAX II trial, 68 patients had SYNTAX score II calculations performed with both invasive coronary angiography (ICA) and coronary MSCT. The mean difference in SYNTAX score II PCI between ICA and coronary MSCT was 0.5±2.1 (limits of agreement –3.8 to 4.7). The intraclass correlation coefficient was 0.97 (95% CI: 0.95-0.99) (unpublished data).
PHILOSOPHY OF THE SYNTAX III REVOLUTION TRIAL
Since its introduction into clinical practice, there has been speculation regarding the possibility of coronary MSCT replacing ICA for the diagnosis of CAD and for the guidance of revascularisation procedures. The prospect of sending a patient to surgery based on a purely non-invasive assessment could potentially decrease medical complications as well as hospitalisation days and, ultimately, costs related to medical treatment.
In addition, coronary MSCT has proved to have a role, especially in low- to moderate-risk patients with suspected CAD, for both diagnosis and prognosis assessment8,31. Its role in evaluating more complex patients with known extensive multivessel disease is still somewhat unclear.
Study objectives
STUDY PURPOSE
The purpose of the planned SYNTAX III Revolution trial is to investigate whether a Heart Team decision-making process regarding the choice of revascularisation strategy based on non-invasive coronary MSCT assessment of CAD with the Revolution CT scanner (GE Healthcare, Little Chalfont, United Kingdom) is equivalent to the standard-of-care assessment based on ICA in patients with multivessel coronary disease. The Heart Team decision-making process will be compared between the two modalities of assessment (invasive vs. non-invasive) regarding the chosen revascularisation strategy (PCI or CABG), the number of vessels requiring treatment and the coronary segments in need of revascularisation.
STUDY DESIGN
The present study is an investigator-initiated, prospective, multicentre, multinational, randomised feasibility clinical trial with an all-comers design. It will randomise two Heart Teams to make a decision between surgical and percutaneous treatment according to either an invasive conventional angiography or a non-invasive multislice CT angiography assessment in patients with de novo three-vessel or left main disease (isolated or in association with one, two or three-vessel disease).
For each patient, the Heart Team (composed of an interventional cardiologist, cardiac surgeon, cardio-radiologist and study coordinator) will be randomly assigned to an “angiography-first” or an “MSCT-first” decision-making algorithm. There will be two independent Heart Teams per hospital and all patients will be assessed by both teams in a crossover design. The final decision will result from a consensus in case of any initial discrepancies (Figure 1).
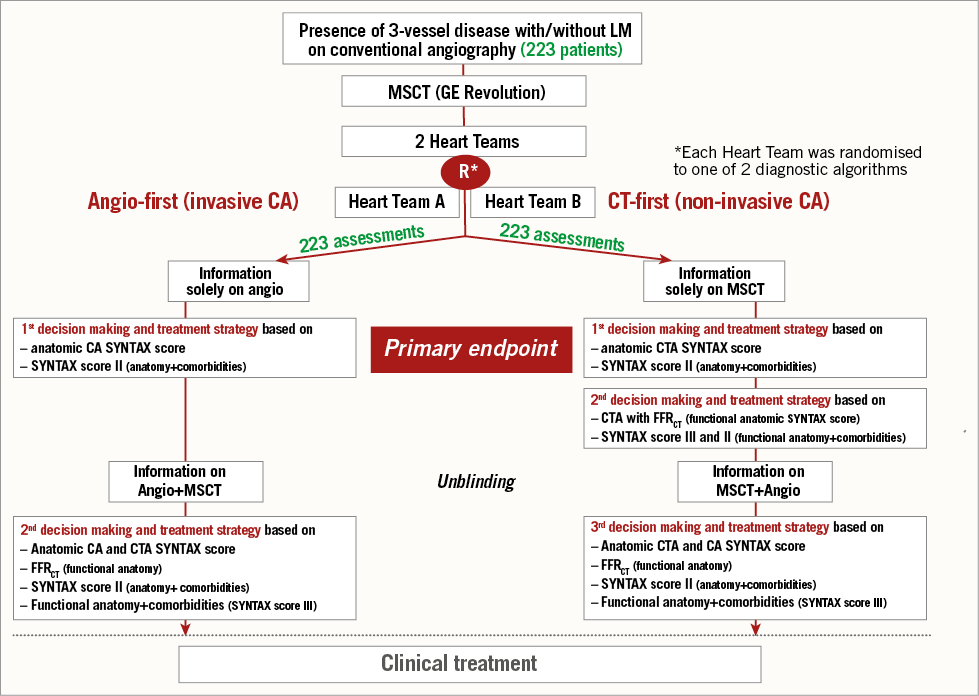
Figure 1. Study flow chart.
ANGIOGRAPHY-FIRST ALGORITHM
Patients will be assessed initially by standard-of-care ICA. The anatomic SYNTAX score and SYNTAX score II will be calculated to guide the selection of the revascularisation strategy. The Heart Team will then record the recommendation of revascularisation modality, number of lesions to be treated and localisation of diseased coronary segments needing treatment. After the recording of the first decision, the additional information of MSCT will be disclosed to the Heart Team and the second decision will then be recorded based on the information derived from both imaging modalities.
MSCT-FIRST ALGORITHM
The same steps as in the “angiography-first” algorithm will be followed by a second Heart Team assessing MSCT images. For both assessments, SYNTAX scores calculated by an independent core laboratory (Cardialysis BV, Rotterdam, The Netherlands) will be made available for consultation.
The first and second decisions, the actual treatment received by the patient and procedure details (arterial segments, number of lesions, etc.) will be recorded for analyses (Figure 1).
PRIMARY ENDPOINT
The primary endpoint of the trial will be the level of agreement, according to Cohen’s kappa, of the initial decision by the Heart Teams on the modality of the revascularisation based on the MSCT-first assessment as compared to the angiography-first evaluation. The Heart Team decision will be made according to the following options:
CABG-only
1. CABG-only. The patient should be treated by CABG due to the high four-year mortality of PCI according to the therapeutic recommendation of the SYNTAX score II.
PCI-only/equipoise
1. Equipoise. The patient could be treated by either CABG or PCI, considering that the four-year mortality predictions are similar for PCI and CABG.
2. PCI-only. Patient should be treated by PCI due to the high four-year mortality of CABG according to the therapeutic recommendation of the SYNTAX score II.
Notably, the Heart Team can overrule the SYNTAX score II therapeutic recommendation whenever it identifies significant additional clinical risk factors not contemplated in the calculation of the SYNTAX score II.
SECONDARY ENDPOINTS
The secondary endpoints include the following: 1) level of agreement in the decision-making strategy based on CT only without functional assessment and the decision-making strategy based on CT with functional assessment (“CT-first” algorithm group); 2) level of agreement in the decision-making strategy based on CT only (with functional assessment) and the decision-making strategy based on CT with functional assessment and conventional angiography (“CT-first” algorithm group); 3) level of agreement in the decision-making strategy based on conventional angiography only and the decision-making strategy based on CT with functional assessment and conventional angiography (“angio-first” algorithm group); 4) inter-rater agreement on revascularisation strategy (based on conventional angiography and CT with functional assessment) of two Heart Teams using an “angio-first” algorithm or a “CT-first” algorithm; 5) anatomical SYNTAX score calculation based on non-invasive CT (visual by Heart Team involving a radiologist) and the resulting SYNTAX score II; 6) anatomical SYNTAX score calculation based on non-invasive CT (visual by core lab) and the resulting SYNTAX score II; 7) anatomical SYNTAX score calculation based on invasive angiography (visual by Heart Team) and the resulting SYNTAX score II; 8) anatomical SYNTAX score calculation based on invasive angiography (visual by core lab) and the resulting SYNTAX score II; 9) CT-based functional SYNTAX score (FFRCT); 10) concordance in SYNTAX score(s) between and within strategies; 11) agreement in coronary stenosis segments to be revascularised between and within strategies.
Other important information such as radiation exposure data for both MSCT and invasive angiography will be collected and compared.
Study methods
PATIENT POPULATION
The population of the trial will comprise patients with de novo three-vessel disease (diameter stenosis [DS] ≥50%) with or without left main stem involvement able to undergo coronary MSCT. Similar to the SYNTAX I and II studies, in the SYNTAX III Revolution trial, patients with any anatomic SYNTAX score are eligible for screening and inclusion in the study.
INCLUSION CRITERIA
Inclusion criteria include the following: 1) patients with at least one stenosis (de novo lesions with DS ≥50% determined by angiographic visual assessment) in all three major epicardial territories (LAD and/or side branch, LCX and/or side branch, RCA and/or side branch) supplying viable myocardium with or without left main stem involvement; 2) patients with hypoplastic RCA with absence of right posterior descending artery and presence of a lesion in the LAD and LCX territories may be included in the trial as a three-vessel disease equivalent; 3) vessel size should be at least 1.5 mm in diameter as visually assessed in diagnostic angiogram; 4) patients with stable (Canadian Cardiovascular Society Class I, II, III or IV) or unstable (Braunwald class IB, IC, IIB, IIC, IIIB, IIIC) angina pectoris and ischaemia; patients with atypical chest pain or those who are asymptomatic provided that they have myocardial ischaemia (e.g., treadmill exercise test, radionuclide scintigraphy, stress echocardiography); 5) patients who have been informed of the nature of the study and agreed to its provisions and have provided written informed consent as approved by the ethics committee of the respective clinical site.
EXCLUSION CRITERIA
Candidates will be deemed ineligible for enrolment in the study if any of the following conditions apply: age <18 years; inability to give informed consent; known pregnancy at the time of enrolment; female of childbearing potential (and last menstruation within the last 12 months) who are not taking adequate contraceptives; female who is breastfeeding at the time of enrolment; prior PCI or CABG; evidence of evolving or ongoing acute myocardial infarction (AMI) in ECG and/or elevated cardiac biomarkers (according to local standard hospital practice) that have not returned to within normal limits at the time of procedure; concomitant cardiac valve disease requiring surgery (reconstruction or replacement); one- or two-vessel disease (at the time of Heart Team consensus); atrial fibrillation; known allergy to iodinated contrast media; glomerular filtration rate below 60 mL/min; heart rate >90 beats/min; participation in another trial with an investigational drug or device.
CORONARY CT ANGIOGRAPHY
In the SYNTAX III Revolution trial, all coronary MSCT imaging will be obtained using a 256 slice CT scanner (Revolution CT; GE Healthcare). This device applies a technique based on a whole heart single beat image acquisition that utilises 160 mm of coverage with 0.28 seconds rotation speed32. Acquisitions will be performed after administration of nitrates with an ECG-triggered one beat scan mode. Beta-blockers are recommended for heart rates >60 beats/min.
ANATOMIC SYNTAX SCORE, SYNTAX SCORE II AND FUNCTIONAL ANATOMY (FFRCT)
The baseline anatomical SYNTAX score for angiography and for MSCT and SYNTAX score II will be calculated by site investigators prior to the start of the Heart Team meeting(s) and will be recorded in the eCRF. The diagnostic angiograms along with acquired and reconstructed MSCT images should be transferred automatically to the independent core lab and to HeartFlow Inc. for the functional assessment (FFRCT). SYNTAX scores calculated by the core lab will be made available to the investigators for consultation for the primary provisional decision-making recording (primary endpoint). Only after this decision is recorded in the eCRF for the primary endpoint of the trial will FFRCT data be made available for site investigators for their final treatment decision. The impact of FFRCT on the final treatment decision will be assessed as a pre-specified secondary endpoint.
RANDOMISATION
Before the start of enrolment, two Heart Teams (A and B) will be formed and registered at each site, including at least one physician from each subspeciality (i.e., radiologist, cardiac surgeon and interventional cardiologist). When a patient is enrolled, the algorithm of decision making is randomly allocated to two Heart Teams (i.e., Heart Team A: CT-first, Heart Team B: angiography-first, or Heart Team A: angiography-first, Heart Team B: CT-first) in order to ensure that the two Heart Teams experience both decision-making processes equally (e.g., CT-based or angio-based). According to the allocated sequence, each Heart Team will discuss and make a treatment decision independently (blinded to the decision made by the other Heart Team). In case any member of the Heart Team is not available due to logistical (e.g., on-call) or other reasons (e.g., pre-exposure to angiography or MSCT), the Heart Team can still proceed with the discussion, as long as at least one physician from all three specialities is available.
Statistical considerations
RATIONALE FOR SAMPLE SIZE CALCULATION
Patients will be randomly allocated to an “angiography-first” or to an “MSCT-first” Heart Team assessment. The Heart Teams will decide on treatment strategy according to the options of “CABG-only” or “equipoise/PCI-only” as described above.
The level of agreement between the two algorithms will be assessed by the kappa value. A kappa value of 0.60 to 0.80 is considered to reflect substantial agreement and a kappa value greater than 0.80 is considered almost perfect agreement between two ratings33. We expect the two Heart Team decisions to reach almost perfect agreement (kappa ≥0.80)33. The trial will be considered to be positive, i.e., substantial agreement between the two diagnostic modalities, if the lower bound of the 95% two-sided confidence interval for the kappa value is ≥0.6.
Based on analyses of the current screening process of the ongoing SYNTAX II trial (unpublished data), it was assumed that the standard-of-care “angio-first” algorithm (control group) would result in a recommendation of “CABG-only” in approximately 30% of the study population and hypothesised that approximately the same rate would be found in the “MSCT-first” algorithm group.
Assuming that both diagnostic algorithms result in a “CABG-only” consensus in 30% of the patients with a kappa of 0.80, a sample size of 200 patients will be sufficient for achieving 90% power to reach a positive trial34. Assuming an attrition rate to account for non-analysable cases of 10%, a total of 223 patients will be included in the study.
Conclusions
The SYNTAX III Revolution trial will be the first prospective, randomised trial to investigate the role of a decision-making process regarding the revascularisation strategy based on non-invasive imaging with coronary MSCT in patients with complex multivessel disease. In addition, automatic non-invasive assessment of functional coronary anatomy complexity will also be evaluated with the FFRCT for functional SYNTAX score on MSCT.
| Impact on daily practice The SYNTAX III Revolution trial will answer a very important clinical question about the role of a non-invasive MSCT assessment alone to select the revascularisation strategy for patients with multivessel coronary disease. |
Appendix. SYNTAX III Revolution trial investigators
Universitair Ziekenhuis Brussel, Brussels, Belgium: Jeroen Sonck; Mark La Meir; Johan De Mey; Kristof De Smet; Danny Schoors; Peter Kayaert; Dries Belsack; Jens Czapla; Stijn Lochy; Jean-Francois Argacha; Kaoru Tanaka; Jan Nijs; Marcel Van Der Linden. Centro Cardiologico Monzino, I.R.C.C.S., University of Milan, Italy: Daniele Andreini; Antonio Bartorelli; Francesco Alamanni; Gianluca Pontone. Centre Cardiologique du Nord, Saint Denis, France: Laurent Macron; Nicolas Bonnet; Philippe Guyon; Jean-Louis Sablayrolles; Franck Digne; Patrick Mesnildrey, David Attias. Centre Hospitalier Universitaire de Nancy, Nancy, France: Damien Mandry; Edoardo Camenzind; Juan-Pablo Maureira. University Hospital Zurich, Zurich, Switzerland: Thomas Lüscher; Francesco Maisano; Philipp Kaufmann; Oliver Gaemperli; Ronny Buechel; André Plass.
Guest Editor
This paper was guest edited by Stephan Achenbach, MD; University of Erlangen, Erlangen, Germany.
Funding
The SYNTAX III trial is an investigator-driven study sponsored by the European Cardiovascular Research Institute (ECRI). For this study, the ECRI received research grants from GE Healthcare and HeartFlow Inc.
Conflict of interest statement
B. Thomsen is an employee of GE Healthcare. C. Rogers is an employee of HeartFlow Inc. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

