Introduction
Drug eluting stents (DES) have dramatically improved the effectiveness of coronary stenting since the introduction of Cypher® in 2002. Cypher® demonstrated that controlled stent-based delivery of sirolimus, a potent cell-cycle inhibitor and anti-inflammatory agent, could significantly reduce late lumen loss and restenosis rates when compared to bare metal stents1. These benefits were observed for multiple patient and lesion categories and have been sustained for more than five years2. Taxus® (Boston Scientific, Natick, MA, USA), Endeavor® (Medtronic, Minneapolis, MN, USA) and Xience® (Abbott Laboratories. Abbott Park, Il, USA) followed the introduction of Cypher® (Cordis Corporation, Bridgewater, NJ, USA) and have also shown significant reductions in restenosis in controlled clinical trials3-5. These FDA-approved DES share several common design features: (a) a metallic scaffold, (b) a durable, strut-adherent polymer coating, and (c) an elutible antiproliferative agent to reduce neointimal hyperplasia.
The latest advance in DES technology and successor to Cypher® is the NEVO™ sirolimus-eluting coronary stent. The primary changes made transitioning from Cypher® to NEVO™ are shown in Table 1. NEVO™ is produced from L605 cobalt chromium alloy and has a thin-strut (<100 µm), open-cell configuration for improved vessel conformability and deliverability. NEVO™ was completely redesigned from the original Conor CoStar® stent and will be available in a full range of lengths (8-38 mm) and diameters (2.25-4.0 mm). The NEVO™ platform is distinct from all other drug-eluting stents because it uses a bioresorbable polymer and uniformly spaced reservoirs embedded in the struts (RES Technology™) to contain the drug/polymer matrix instead of a conformal polymer coating overlaying the struts (Figure 1). Reservoirs are able to maintain their proper orientation to the vessel during expansion through a design feature known as a ductile hinge which can accommodate all of the mechanical strain generated during stent expansion.
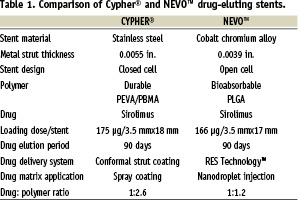
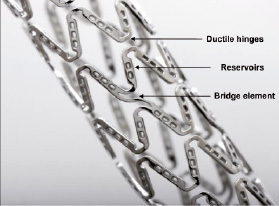
Figure 1. NEVO™ sirolimus-eluting coronary stent.
RES Technology™
RES Technology™ delivers drugs from uniform cylindrical structures, only one nanolitre in volume, that span the entire thickness of the strut. Each NEVO™ stent contains several hundred reservoirs in proportion to the diameter and length of the stent. Reservoir based drug delivery offers many potential advantages over conventional DES coatings (Table 2). Reservoirs protect the drug-polymer matrix from mechanical disruption during passage through highly calcified lesions that can cause delamination of polymer coatings. Reservoirs allow higher drug loading capacity and higher drug/polymer ratios because polymers recessed in reservoirs are not subject to the elongation and deformation of polymer coatings. Reservoirs also require less polymer mass than conventional surface coatings and can reduce strut thickness by 10-30 µm (Figure 2). Reservoirs may offer better vessel biocompatibility by providing a stent surface that is predominantly bare metal with virtually no polymer contacting the vessel wall on implantation. Finally, RES Technology™ expands the therapeutic potential of DES by making it possible to deliver multiple drugs from a stent with independent release profiles and to treat the metallic surface of the stent without affecting its drug/polymer attributes.

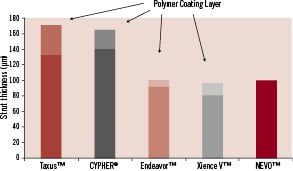
Figure 2. Total strut thickness for NEVO™ and other drug eluting stents.
The RES Technology™ filling process uses a high-speed, optically-guided, nanodroplet injection system to load a drug-polymer-solvent formulation into the reservoirs. The formulation and jetting process are precisely controlled to deliver multiple injections of uniform droplet size. Cycles of reservoir filling and drying occur until the desired drug load is attained. This process enables a wide range of reservoir inlay designs that result in uniform control of release kinetics, including first order, burst and delayed release profiles.
NEVO™ biodegradable polymer technology
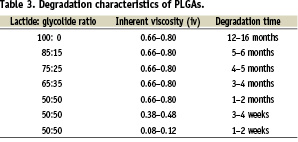
The non-degradable polymers used in the coatings for Cypher®, Taxus®, Endeavor®, and Xience® permanently remain in the vessel wall after the drug has inhibited neointimal proliferation. Since the polymer serves no function after drug delivery, it is logical to use a biodegradable polymer whose resorption is tuned to the desired release profile of the drug. NEVO™ uses a biodegradable polymer system to control the release of sirolimus, which represents an important advance in DES technology. Poly-lactic acid-co-glycolic acid (PLGA), is a well-recognised biodegradable polymer that has been used in a wide range of biomedical applications, including absorbable sutures, orthopaedic devices and drug-delivery implants6. PLGA is formed by co-polymerisation of monomers of D,L-lactic acid and glycolic acid. In the body, PLGA is degraded by hydrolysis of ester linkages to release lactic acid and glycolic acid residues that are subsequently metabolised via the Kreb’s cycle to carbon dioxide and water.
The rate of PLGA degradation can be controlled by altering the lactide:glycolide (L:G) ratio and the molecular weight (often expressed as the inherent viscosity, iv) of the copolymer (Table 3). PLGA degradation times can range from weeks to years. The fastest degradation rates occur when the L:G molar ratio is 50:50 and slows as the L:G ratio increases above 50:50, principally due to the slower rate of hydrolysis of poly-lactide residues. In addition, lower molecular weight polymers resorb more quickly because they require less hydrolysis to generate water-soluble oligomers that can diffuse out of the bulk matrix. They also allow more rapid ingress of water into the matrix to accelerate bulk hydrolysis7.
NEVO™ formulation and release kinetics
NEVO™ was designed to have similar sirolimus content and pharmacodynamics as Cypher®. A 3.5 mmx17 mm NEVO™ stent nominally contains 166 µg of sirolimus. For comparison, a 3.5 mmx18 mm Cypher® stent nominally contains 175 µg of sirolimus. The dose of sirolimus in NEVO™ is proportional to stent diameter and length and is based on the total number of reservoirs.
The reservoir design of NEVO™ involves a base + drug matrix configuration (Figure 3). The base is composed predominantly of PLGA (75:25) and functions as a luminal-side barrier so that sirolimus is efficiently released into the vessel wall. The drug matrix is composed of sirolimus blended with PLGA in a formulation that controls release and delivers therapeutic levels of sirolimus to the artery wall for approximately 90 days.
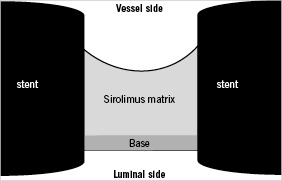
Figure 3. Cross section of a NEVO™ reservoir showing base/matrix inlay design.
Sirolimus release profiles for NEVO™ and Cypher® were obtained from stents implanted into normal porcine coronary arteries (Figure 4). While sirolimus release from Cypher® occurs by diffusion from all surfaces, drug release from NEVO™ is more complex and involves both diffusion and bulk erosion of the polymer matrix within the reservoirs. Despite these differences, the results show that NEVO™ achieves controlled drug elution with approximately 80% of sirolimus released over a period of 30 days and 100% eluted over 90 days. This pattern of release was tailored so that sirolimus arterial content in the stented vessel segment closely matches Cypher® (Figure 5).
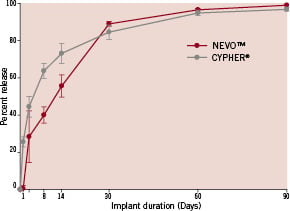
Figure 4. In vivo sirolimus elution profile for NEVO™ and Cypher® stents implanted in porcine coronary arteries.
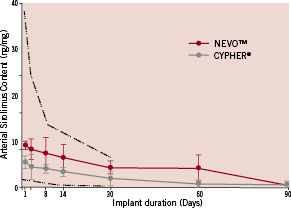
Figure 5. Sirolimus tissue concentrations for NEVO™ and Cypher® stents implanted in porcine coronary arteries out to 90 days. The dotted-dashed lines represent the minimum and maximum arterial sirolimus content historically achieved with Cypher® stents in the porcine coronary artery model.
Explanted stents harvested at periodic intervals from porcine coronary arteries show time-dependent erosion of the drug polymer inlay with complete resorption in about 90 days (Figure 6). Thus, in contrast to current DES with permanent polymer coatings, NEVO™ returns to a bare metal stent in about three months. This may have important implications regarding long-term biocompatibility of NEVO™ and the potential to reduce extended dual antiplatelet therapy (DAPT) proactively in some patients, and reactively in any patient when clinically necessary (e.g., bleeding or need for urgent surgery).

Figure 6. Time-dependent biodegradation of NEVO™ reservoir matrix implanted in porcine coronary arteries and explanted at 8, 30, 60 and 90 days.
NEVO™ preclinical histology
NEVO™ stents were implanted along with bare metal reservoir control stents (BMS) into porcine coronary arteries (10% overstretch) to assess safety and efficacy. Stents were explanted for histological analysis at 30, 90 and 180 days. Representative sections for NEVO™, and BMS are shown in Figure 7. NEVO™ exhibited reduced neointimal hyperplasia and inflammation (P<0.05) when compared to BMS at 30 days. At 90 and 180 days, there were no significant differences between the groups as is the case for all DES in this model. Re-endothelialisation of NEVO™ was rapid and complete in single or overlapped stents by 30 days based on endothelialisation scores and scanning electron microscopy (Figure 8). These data demonstrate that the combination of stent, polymer, and drug embodied in NEVO™ has excellent tissue biocompatibility through six months, and leads to early re-endothelialisation, reduced neointimal hyperplasia, and low peri-strut inflammation.
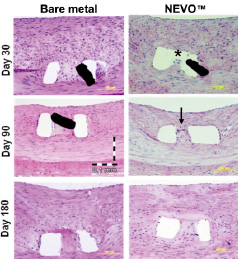
Figure 7. Representative cross sectional histology of NEVO™ and bare metal RES technology™ stents explanted from porcine coronary arteries at 30, 90 and 180 days. Sections were obtained through reservoirs and show the space occupied by PLGA-sirolimus matrix in NEVO™ at 30 days (denoted by asterisk) with complete ingrowth of neointima at 90 days (black arrow). The black trapezoidal objects are strut artifacts.
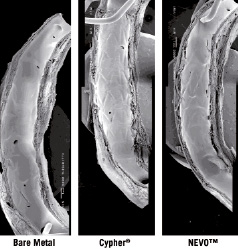
Figure 8. Representative scanning electron micrographs of prototype NEVO™, Cypher® and bare metal RES Technology™ stents implanted in porcine coronary arteries for 30 days.
NEVO™ clinical programme
A worldwide clinical programme is underway to assess the safety and effectiveness of the NEVO™ sirolimus-eluting coronary stent (Table 4). Three trials will evaluate NEVO™ performance versus the “gold-standard” Cypher®, the current market leader, Xience®, and the most common comparator in DES trials, Taxus®. The first study, NEVO RES-1, is a 1:1 randomised clinical trial (RCT) comparing NEVO™ to the Taxus-Liberte® paclitaxel-eluting coronary stent in single de novo native coronary artery lesions of 2.5-3.5 mm reference vessel diameter and <28 mm in length. A total of n=394 patients were enrolled at 40 centres in Europe, South America, Australia and New Zealand. The primary endpoint was angiographic in-stent late lumen loss at six months with secondary clinical endpoints of TLR, TVR, TVF, MACE and stent thrombosis. All patients received aspirin and clopidogrel for at least six months.
The six month results of NEVO RES-1 were presented at EuroPCR 20098. While the trial was designed to demonstrate noninferiority of NEVO™ to TAXUS Liberte®, the prespecified analysis actually demonstrated both non-inferiority and superiority of NEVO™ over Taxus® with respect to six month in-stent late lumen loss (LL) (NEVO™, n=180, LL=0.13±0.31 mm; Taxus®, n=162, LL= 0.36±0.46 mm; P<0.001). All other angiographic and clinical endpoints, either statistically or numerically favoured NEVO™. Several subgroups, including diabetic patients, for whom randomisation was stratified, showed similar findings. No stent thromboses were reported for NEVO™, while two stent thromboses, despite ongoing DAPT, were reported for Taxus Liberte®. Publication of these findings will include prespecified IVUS and quality-of-life substudy outcomes. Long term clinical follow-up will continue through five years.
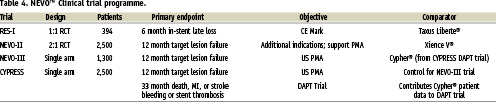
NEVO-II is a multicentre trial that will randomise “all-comers” undergoing PCI in a 2:1 fashion to NEVO™ or Xience V® everolimus-eluting coronary stents. Approximately 2,500 patients will be enrolled at more than 100 centres worldwide, excluding the US and Canada. This trial is a head-to-head comparison of safety and efficacy outcomes in a full spectrum of patient and lesion subgroups undergoing catheter-based intervention and will also provide the long term clinical data for NEVO™ that is required for regulatory submission. The primary endpoint of NEVO-II is target vessel failure (TVF) at 12 months. Principal investigators for the trial are Drs. Patrick Serruys, Stephan Windecker and Manuel Sabaté.
NEVO-III is the pivotal clinical trial for NEVO™ in the US. It will be conducted as a single-arm non-randomised study in ~1,300 patients at 50 sites in the US and Canada. The comparative stent for Nevo-III will be Cypher® and control data will come from the CYPRESS trial, a non-randomised US study of Cypher® that was initiated in August 2009. The CYPRESS trial was started before NEVO-III to allow patients also to be randomised at 12 months into the DAPT trial, an HCRI-lead study comparing 12 months versus 30 months of dual antiplatelet therapy after percutaneous coronary intervention with approved DES or BMS. Sites enrolling in CYPRESS will transition to enrolling in NEVO-III so that sequential enrolment of similar patient populations will occur in the two trials. NEVO-III will enrol patients with up to two lesions in one or two vessels (2.25–3.5 mm diameter vessels, maximum lesion length <34 mm, maximum implanted stent length <66 mm). Primary endpoints for Nevo-III and CYPRESS include target vessel failure (TVF) at 12 months. CYPRESS also has a prespecified secondary safety endpoint at 33 months, as part of the larger DAPT trial. For both Nevo-III and CYPRESS, Dr. Dan Simon will be the principal investigator and Dr. David Kandzari will be the co-principal investigator.
Future directions with RES Technology™
RES Technology™ provides an ideal stent platform for delivery of therapeutic agents. Among its advantages are: (a) deep recessed reservoirs for expanded drug loading capacity, (b) potential to deliver multiple drugs, (c) independent control of release kinetics, (d) directional control of drug release (toward the lumen or vessel wall), and (e) potential to modify the bare metal surface for additional therapeutic benefit. An example of the drug delivery options afforded by RES Technology™ is illustrated in Figure 9.
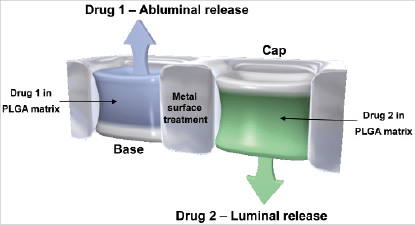
Figure 9. Example of a dual drug eluting stent with directional release.
Cordis is exploring therapeutic programs in several key areas:
(a) Thrombosis – Despite a relatively low frequency of occurrence, stent thrombosis remains a serious issue for both bare metal and drug eluting stents. It is thought that drug eluting stents may delay re-endothelialisation and thereby necessitate a longer regimen of aspirin/clopidogrel than BMS. A recently published large meta analysis supports the thesis that premature withdrawal of clopidogrel is an important predictor of DES thrombosis9. In addition, idiosyncratic reactions to stent components, including permanent polymer coatings, may lead to hypersensitivity responses and inflammation that could play a role in very late stent thrombosis10.
NEVO™ directly addresses many of these biocompatibility concerns because it: (1) has less polymer mass than conventional DES, (2) essentially eliminates initial polymer contact with surrounding tissue through recessed reservoirs, and (3) makes the entire drug/polymer matrix fully resorbable in approximately three months so that the stent returns to bare metal. RES Technology™ also makes it possible to co-elute a potent antithrombotic agent to block coagulation or platelet aggregation locally at the interface between the stent and flowing blood. In addition, the predominantly bare metal surface of NEVO™ can be modified with an active surface coating to reduce stent thrombogenicity (e.g. covalently bound heparin) or to accelerate re-endothelialisation (e.g. covalently bound peptides that attract endothelial progenitor cells). These NEVO™-based strategies offer the hope of further reducing early, late, and very late stent thrombosis as well as reducing the currently recommended long term requirement for clopidogrel.
(b) Acute myocardial infarction – The goal of current therapy for patients with acute myocardial infarction (MI) is to achieve early and complete restoration of coronary blood flow with PCI and a stent. The potential to further reduce infarct size by preventing reperfusion with an adjunctive therapeutic agent is a rational strategy that could reduce morbidity and mortality. RES Technology™ can be used to elute a potent drug regionally over a period of 24-72 hours post stent deployment to reduce reperfusion injury to the ischaemic myocardium. The potential for RES Technology™ to provide a new standard of treatment in acute MI and take DES in a new therapeutic direction is an attractive possibility. Novel therapeutic agents for reperfusion injury offer hope that this strategy can be successful11.
(c) Diabetes – Diabetes is a complex metabolic disorder that exacerbates coronary artery disease. Diabetics have more complex, diffuse disease and increased neointimal tissue growth after stenting12. Although Cypher® produces dramatic reductions in LL and TLR in diabetics, compared to bare metal stents, the response is still higher than in nondiabetic patients13 and provides a rationale for new strategies to improve treatment outcomes. Complementary therapeutic agents that synergise with sirolimus to further reduce neointimal hyperplasia may be beneficial in diabetics. RES technology™ offers the ideal platform to explore a complementary dual-drug strategy to further improve outcomes in this challenging population.
Summary
NEVO™ with RES Technology™ is a major advance in DES that couples a thin-strut cobalt chromium stent with reservoir-based delivery of sirolimus in a fully bioresorbable polymer. It offers competitive deliverability and scaffolding strength coupled with precise, controlled release of sirolimus. Unlike other DES, NEVO™ provides a predominantly bare metal interface with the artery wall on implantation, and after completely eluting sirolimus, becomes a fully bare metal stent in only 90 days. Preclinical studies show that this combination produces rapid healing and complete re-endothelialisation with very low peri-strut inflammation out to six months. In the RES-1 clinical trial, NEVO™ has shown remarkably consistent Cypher-like angiographic results at six months, with significantly lower late lumen loss than Taxus Liberte®, low MACE and no incidents of subacute or late stent thrombosis. Longer term follow up (one to five years) is planned, as are additional clinical trials to establish the broad utility and safety of NEVO™.
Beyond NEVO™, RES technology™ offers the unique ability to deliver multiple therapeutic agents from a stent to enhance the effectiveness of sirolimus. Additional drugs, with local or regional activity, may potentially be used to reduce stent thrombosis, improve efficacy in diabetics, reduce infarct size, or treat vulnerable plaque. This platform has the potential to expand our treatment strategies and provide new solutions for patients with cardiovascular disease.

