Abstract
Aims: To compare the frequency and extent of Troponin I and late gadolinium enhancement (LGE) cardiac magnetic resonance (CMR) defined injury following PCI compared with CABG in patients with multivessel and/or left main coronary artery disease (CAD), and interpret these finding in light of the new ESC/ACCF/AHA/WHF Task Force definitions for necrosis and infarction.
Methods and results: Prospective, registered, single centre randomised controlled trial. Eighty patients with 3 vessel CAD (≥50% stenoses), or 2 vessel CAD including a type C lesion in the LAD, and/or left main disease were enrolled. Mean SYNTAX and EuroSCOREs were similar for both groups. Forty patients underwent PCI with drug eluting stents and 39 underwent CABG (one died prior to CABG). In the PCI group 6/38 (15.8%) patients had LGE, compared with 9/32 (28.1%) CABG patients (p=0.25). Using the new Task Force definitions, necrosis occurred in 30/40 (75%) PCI patients and 35/35 (100%) CABG patients (p=0.001), whilst infarction occurred in 30/40 (75%) PCI patients and 9/32 (28.1%) CABG patients (p=0.0001).
Conclusions: Periprocedural necrosis according to the Task Force definition was significantly lower in the PCI group, and universal in the CABG group. The incidence and extent of CMR defined infarction following PCI did not differ compared with CABG. This demonstrates that PCI can achieve revascularisation in complex patients without increased procedural myocardial damage.
Introduction
Increased cardiac biomarkers following coronary revascularisation was first noted with coronary artery bypass grafting (CABG) in the 1970s1-3. Since then, periprocedural myocardial injury following CABG and percutaneous coronary intervention (PCI) has been studied extensively. Studies utilising postprocedural cardiac magnetic resonance imaging (CMR) have shown that following CABG or PCI, larger elevations of creatine kinase-MB (CK-MB)4 and troponin I5,6 reflect the absolute mass of new irreversible myocardial necrosis. The incidence of CMR defined periprocedural myocardial injury following PCI is as high as 28% following complex PCI5, and up to 36-44% following CABG6. However no study has directly compared which revascularisation method leads to a greater amount of periprocedural injury as defined by CMR.
Recently the definitions of periprocedural myocardial necrosis and infarction were redefined by the joint ESC/ACCF/AHA/WHF task force7. Following PCI or CABG in patients with normal baseline troponin values, elevations of cardiac biomarkers above the 99th percentile upper reference limit (URL) define periprocedural myocardial necrosis. The definitions of myocardial infarction differ however. Following PCI, biomarker elevation greater than 3 x 99th percentile URL defines PCI-related infarction, whilst CABG requires an increase greater than 5 x 99th percentile URL, plus one of the following; new pathological Q waves, new LBBB, angiographically documented new graft or native vessel occlusion, or imaging evidence of new loss of viable myocardium.
Our aim was to compare the frequency of periprocedural myocardial injury following CABG compared with PCI for patients with triple vessel and/or left main disease assessed by troponin I, CK-MB and CMR, and to evaluate these findings in light of the new definitions from the joint ESC/ACCF/AHA/WHF task force.
Methods
Study design
The Myocardial Injury following Coronary Artery Surgery versus Angioplasty (MICASA) trial is a prospective, single blinded, single centre, randomised (1:1) trial of myocardial injury following percutaneous coronary intervention (PCI) compared with coronary artery bypass grafting (CABG). The study is registered online as: ISRCTN25699844: http://www.controlled-trials.com/ISRCTN25699844.
Ethics
The study was approved by our institutional ethics committee and informed written consent was obtained from each patient.
Selection and randomisation of patients
Patients who underwent coronary angiography at the John Radcliffe Hospital, Oxfordshire, United Kingdom during the 30 months of the study were considered for the trial. Patients with stable angina, unstable angina (Braunwald class 1 or 2), or documented silent ischaemia on functional stress testing were eligible for the study. The interventional cardiologist and cardiac surgeon evaluated eligible patients with previously untreated three vessel coronary artery disease (minimum 50% stenosis in each vessel), or 2 vessel coronary artery disease including an AHA type C lesion in the left anterior descending artery, and/or a functionally significant left main stem stenosis of at least 50%. Diagnostic coronary angiograms were scored according to the Syntax score algorithm8. Patients in whom it was determined that equivalent anatomical revascularisation could be achieved with either PCI or CABG were randomly assigned to undergo one of the two treatment options by means of a computer generated, permuted block randomisation method. Treatment assignment was concealed until randomisation by use of a password protected computer program. The allocation sequence was generated by non-study personnel, and patients were enrolled and assigned to their treatment group by study personnel other than those performing revascularisation.
Patients were excluded from the study if they had any contra-indication to aspirin or clopidogrel, were women of child bearing potential, had no viable myocardium in the area subtended by diseased vessels, required concomitant cardiac surgery and/or presented with an acute myocardial infarction or Braunwald class 3 unstable angina. From 14th March 2007, patients were also excluded from the study if they had a calculated glomerular filtration rate of ≤60 mls/min, due to concerns over gadolinium administration in patients with renal impairment.
Treatment and procedures
Patients undergoing PCI were pre-treated with aspirin 75 mg once daily for at least two days and clopidogrel 300 mg at least six hours prior to PCI. Heparin was given intravenously to all patients prior to PCI at a dose according to operator discretion. Upfront weight adjusted glycoprotein IIb/IIIa inhibitor (abciximab) followed by a 12 hour infusion was the preferred strategy unless contraindications existed. Use of drug eluting stents was the preferred strategy for all lesions. Angioplasty technique (use of adjunctive lesion preparation, direct stenting, post-inflation, and bifurcation technique) was left to operator discretion but care was taken to avoid loss of side branches where possible. All patients underwent PCI in one sitting and there were no staged procedures. Aspirin was prescribed indefinitely for all patients who underwent randomisation. Clopidogrel was prescribed to all PCI patients with an intended duration of at least 12 months.
Patients undergoing CABG ceased all antiplatelet agents at least two days prior to surgery. General anaesthesia was administered according to usual care, with coronary artery bypass using non-pulsatile flow and a membrane oxygenator. Core patient temperature was allowed to drift down to a minimum of 32 degrees Celsius. Myocardial protection was achieved with cold crystalloid cardioplegia delivered antegradely. Use of saphenous vein and/or arterial conduits was left to the discretion of the operator, although all patients received at least one pedicle internal mammary artery graft.
CMR: imaging time points, protocol, post processing and data analysis
Patients were studied at 1.5T (Sonata, Siemens Medical Solutions, Erlangen, Germany). Baseline CMR assessment was performed in the fortnight prior to revascularisation. Repeat CMR was performed seven days (4-10 days) after the procedure, and a third CMR assessment was undertaken six months (5-7 months) after revascularisation. At each time point, left ventricular functional assessment and late gadolinium enhancement (LGE) imaging were performed as previously described5,6.
For analysis of left ventricular function, an experienced observer who was blinded to patient data analysed the short-axis SSFP images using customised software (Argus; Siemens, Erlangen, Germany). For analysis of LGE, two experienced observers who were blinded to patient data interpreted the images, acting in consensus. Areas of LGE were quantified using customised software (MATLAB R2007b; The MathWorks, Natick, MA, USA) with computer-assisted planimetry of short-axis images. New LGE was defined as those pixels with image intensities >2 SDs above the mean image intensity in a user-defined remote myocardial region within the same slice9.
Laboratory markers
Cardiac Troponin I (cTnI) and creatine kinase-MB (CK-MB) were measured before treatment and at 1, 6, 12 and 24 hours after revascularisation. CTnI and CK-MB were quantified with automated chemiluminescent immunoassay techniques on the Siemens ADVIA Centaur and Siemens IMMULITE, respectively (Siemens Healthcare Diagnostics, Frimley, UK). The ADVIA Centaur assay has a 10% imprecision at 0.05 ug/L, with a 99th percentile upper reference limit of 0.06 ug/L.
Endpoints
The primary endpoints were the frequency of new myocardial injury following PCI and CABG as assessed by postprocedural elevation of troponin I and new LGE on postprocedural CMR. Secondary endpoints included the total amount of new myocardial necrosis (in grams) assessed by CMR, the change in left ventricular ejection fraction assessed by CMR, and periprocedural myocardial necrosis and infarction as defined by the new universal definition7.
Statistical analysis
Figures are presented as number (percent) except where specified. For categorical outcomes, Chi Square or Fisher’s exact test was performed where appropriate. For comparison between means, independent T test was used, with equality of variance tested with Levene’s test. For comparison of the means of two variables for a single group, the paired T test was used. Where data was not normally distributed, data is presented as median (range) and results compared using the Mann Whitney U test. The correlation between troponin and necrosis (grams) was measured using Spearman’s rank test. Patients who were excluded from the CMR comparisons were still included in the clinical/biochemical analysis. A probability of P≤0.05 was considered statistically significant.
Power calculation
On the basis of the results observed in previous work by our group, we expected the frequency of periprocedural injury to be 44% in the CABG group.6 On the basis of this, we estimated that we would need 30 patients in each group to detect a 25% difference in the primary endpoint (frequency of periprocedural injury) between the groups with a significance level of 0.05 and a power of 80%.
Results
Patient characteristics
Eighty (80) patients were recruited between 1st June 2006 and 1st December 2008. One patient died after being randomised to CABG, with the remaining 79 patients all undergoing their assigned treatment. No patients were lost to follow-up. In the CABG group, four patients had incomplete biomarkers and were not included in the analysis. There were eight patients who did not undergo a second CMR scan with gadolinium (one PCI patient, seven CABG patients) due to claustrophobia (n=5) or impaired renal function post-revascularisation (n=3). One patient in the PCI group had insufficient image quality to assess LGE (Figure 1).
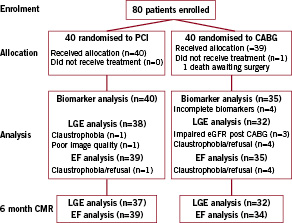
Figure 1. Consort statement flow diagram of recruited patients. PCI: percutaneous coronary intervention; CABG: coronary artery bypass grafting; LGE: late gadolinium enhancement; eGFR: estimated glomerular filtration rate; EF: ejection fraction; CMR: cardiac magnetic resonance imaging
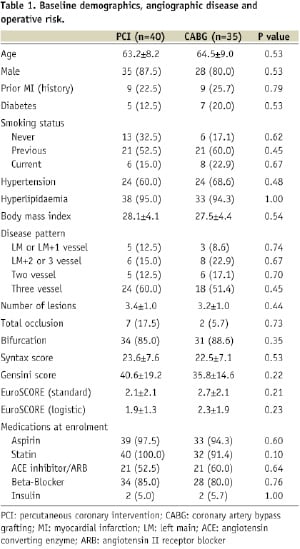
The treatment groups were well matched with respect to the most of the baseline demographic variables (Table 1).
Treatment variables
In the PCI group, 40 patients underwent attempted PCI to 102 vessels (2.55 treated vessels per patient), using 170 stents (1.67 stents per vessel). Revascularisation was completed in 39/40 (97.5%). The procedure was uneventful in 35 patients. Occlusive dissection was successfully treated in three patients and in one patient there was haemodynamic disturbance probably caused by embolism. One patient failed PCI due to inability to cross a chronic total occlusion, and later underwent CABG. Patients undergoing PCI received an average of 4.3±1.6 stents, with an average stent length of 78.6 mm±32.0 mm per patient. All patients received drug eluting stents, either using the Taxus express (Boston Scientific, Natick, MA, USA) paclitaxel stent (n=66), Xience V (Abbot Vascular, Redwood City, CA, USA) everolimus stent (n=62), Promus (Boston Scientific) everolimus stent (n=30) or the Cypher Select (Cordis, Johnson & Johnson, Warren, NJ, USA) sirolimus stent (n=10). Two vessels in separate patients received a bare metal stent. All patients received a heparin bolus (5,000-10,000 I.U.) prior to PCI, with 29/40 (72.5%) also receiving a weight adjusted intravenous abciximab bolus prior to PCI. Of the 29 patients who received abciximab, 27/29 (93.1%) also received a 12 hour infusion following PCI. The average procedure time was 68±28 minutes.
In the surgical group, 39 patients underwent on pump CABG using a total of 99 grafts (2.54 grafts per patient). One patient died after randomisation awaiting surgery. Conduit material used for bypass grafting were; 38 pedicle left internal mammary arteries, one pedicle right internal mammary artery graft, 58 saphenous vein grafts and two free arterial grafts. The average time spent on bypass was 58±19 minutes, with an average cross clamp time of 36±11 minutes.
12-lead electrocardiogram and biomarkers
ECG analysis revealed no patients with new Q wave myocardial infarction following revascularisation. Troponin I and CK-MB elevation were significantly higher following CABG compared with PCI.
Troponin was undetectable before revascularisation in 76/80 (97.5.5%) patients but four patients had minor baseline elevation (0.2, 0.3, 0.5 and 1.5 µg/L, respectively). After PCI the mean peak troponin I was 2.51±5.70 µg/L compared with 10.42±9.07 µg/L in the CABG group in the first 24 hours (p=0.001). The median area under the curve (AUC) for Troponin I was 11.0 (interquartile range 0.9 to 31.0) and 89.2 (interquartile range 61.8 to 214.3) for PCI and CABG respectively (U=152, p=0.0001).
The mean peak CK-MB in the PCI group was 6.04±8.04ng/mL compared with 32.6±23.3 ng/mL in the CABG group in the first 24 hours (p=0.0001). The median AUC for CK-MB was 68.6 (interquartile range 48.4 to 129.6) and 412.3 (interquartile range 244.5 to 626.8) for PCI and CABG, respectively (U=65, p=0.0001).
Late gadolinium enhancement
In the PCI group 6/38 (15.8%) patients had new LGE on the early postprocedure CMR, compared with 9/32 (28.1%) in the CABG group (p=0.25). In those with new LGE, the mean amount of new necrosis (grams) following PCI was 5.6±2.8 compared with 8.7±4.5 following CABG (p=0.12), see Table 2.
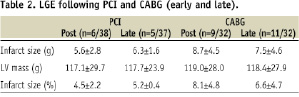
Figure 2 shows individual patient data (troponin I and CK-MB) and the relationship with new LGE.
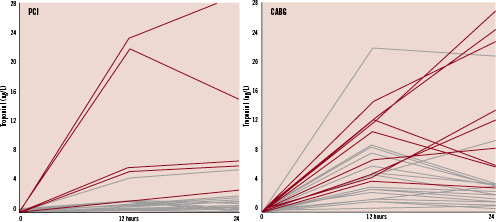
Figure 2. Individual patent data for troponin I at baseline, 12 hours and 24 hours. Red lines indicate new LGE. Black lines indicate no new LGE. The left panel shows data for patients undergoing PCI. One patient whose troponin I peaked above 4.0 ug/L did not have new LGE. This was the only patient who underwent rotational atherectomy. The right panel shows data for patients undergoing CABG. Four patients had troponin I that peaked above 8.0 ug/L at 12 or 24 hours, and did not have new LGE. All of these patients had significant amounts of pre-existing LGE prior to surgery.
Examples of LGE following PCI and CABG are shown in Figures 3 and 4.
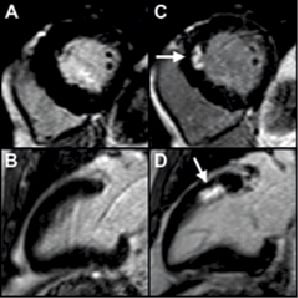
Figure 3. An example of new LGE in a 66-year-old male following PCI for triple vessel disease. Baseline CMR demonstrates normal, viable myocardium (panel A: short axis, and panel B: vertical long axis). During treatment of a complex lesion in the left anterior descending artery, the vessel dissected with transient slow flow. A total of four stents (total stent length 85 mm) were required to restore patency and TIMI III flow. Troponin I peaked at 21.9 ug/L within 24 hours, and the postprocedure CMR demonstrated an area of LGE (arrows) in the anteroseptal wall (panel C: short axis, and panel D: vertical long axis), indicative of irreversible myocardial necrosis equivalent to 8.3 grams of myocardium.
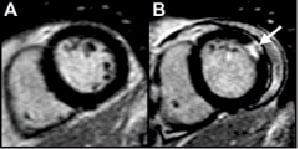
Figure 4. An example of new LGE in a 60-year-old male following CABG for double vessel disease with a dominant circumflex artery. Baseline CMR demonstrates normal, viable myocardium (panel A, short axis). He received a pedicle left internal mammary artery graft to the mid-LAD and a saphenous vein graft to the circumflex artery. Troponin I peaked at 26.9 ug/L within 24 hours, and the post-procedure CMR demonstrated an area of LGE (arrow) in the anterolateral wall (panel B), indicative of irreversible myocardial necrosis equivalent to 13.7 grams of myocardium.
There was a strong positive correlation between the absolute peak troponin I postprocedure and the amount of new myocardial necrosis in grams assessed by CMR, rs=0.57, p=0.01 (Figure 5).
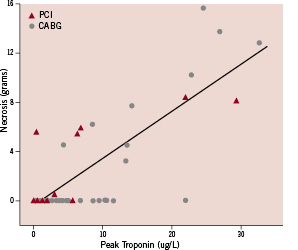
Figure 5. Myocardial necrosis in grams assessed by CMR versus peak troponin I in the first 24 hours following revascularisation. There was a positive correlation between the absolute peak troponin I post-procedure and the amount of new myocardial necrosis in grams assessed by CMR, rs=0.57, p < 0.01.
In the six patients with new LGE following PCI, four had complications during PCI (three dissections with transient slow flow, one angiographically visible embolus). Each of these four patients had elevated troponin (0.4, 6.3, 21.9 and 29.3 µg/L) and evidence of LGE. Of the 32 patients in the PCI group who did not demonstrate new LGE, four patients had peak troponin values of >2.0 ug/L, with two of these having pre-existing necrosis on CMR. The only patient to undergo rotational atherectomy had a troponin of 5.6 µg/L post-PCI, with no LGE discernable on CMR.
Of the 23 patients in the CABG group who did not demonstrate new LGE, all had peak troponin values >2.0 ug/L, with 12 of these patients having pre-existing LGE on their baseline CMR scan. Two patients in the CABG group had very large enzyme elevation postprocedure (2nd and 3rd highest) however were unable to undergo scanning due to claustrophobia. Time spent on bypass was not longer for patients who sustained CMR defined injury, with bypass times of 49±9 versus 58±21 minutes in patients with and without CMR defined injury respectively (p=0.11).
At six months, there was no significant change in the amount of LGE (grams) in those patients who had new LGE following revascularisation. In the 13 patients with new LGE who also had six month follow-up CMR scans, the mean amount of necrosis in grams was 8.2±3.8 following revascularisation, and 7.9±3.8 at six months, p=0.25. LGE at each time point according to revascularisation strategy is shown in Table 2.
Clinical outcomes between treatments at one year were comparable. One year major adverse cardiac and cerebrovascular event rates were 12.5% for PCI and 15.0% for CABG.
Frequency of necrosis and MI by new definition
Using the new universal definition of periprocedural necrosis in those patients with available Troponin I measurements, necrosis occurred in 30/40 (75.0%) PCI patients and 35/35 (100%) CABG patients (p=0.0001). Using the new universal definition of myocardial infarction in those with Troponin measurements (and CMR for the CABG group), myocardial infarction occurred in 30/40 (75.0%) PCI patients and 9/32 (28.1%) CABG patients (p=0.0001). This difference relates to the requirement for the addition of CMR evidence of new LGE to the enzyme data to diagnose CABG related MI. Notably only 6/30 (20.0%) patients in the PCI group who met the new criteria for periprocedural myocardial infarction had LGE on CMR.
Ejection fraction
The baseline ejection fraction (EF, %) in the PCI and CABG groups were 68.8±10.4 and 67.9±10.1 respectively (p=0.70). Regional wall motion abnormalities were seen in 14/15 (93.3%), corresponding in all cases to vascular territories sustaining new areas of LGE. One patient who sustained a small papillary muscle infarct (troponin 0.4 µg/L) following PCI had no new wall motion abnormality. Following revascularisation, the change in EF between groups was not statistically different at one week or six months (two way ANOVA, F=0.024, p=0.88). There was a minor, but significant decline in EF at six months within each treatment group (two way ANOVA, F=4.233, p=0.02).
Relationship between SYNTAX and log EuroSCOREs versus LGE
The SYNTAX and log EuroSCOREs were not predictive of periprocedural myocardial infarction. The mean patient SYNTAX score in patients without new LGE was 23.8±7.4 versus 19.9±5.6 with new LGE (p=0.07). The mean log EuroSCORE in patients without new LGE was 1.9±1.3 versus 2.3±2.4 with new LGE (p=0.37). Furthermore, there was no relationship between peak troponin and SYNTAX or EuroSCOREs (Syntax r=–0.039, p=0.74 and Log Euro r=–0.020, p=0.87).
Discussion
Our study was unable to demonstrate a difference in the rate of CMR defined periprocedural necrosis between PCI and CABG for patients with left main and/or triple vessel disease, most likely due to our insufficient sample size. However, we were able to demonstrate that following CABG patients have significantly higher levels of troponin I and CK-MB in the first 24 hours. We also showed that in both patient groups, postprocedural peak troponin reflects myocardial necrosis measured by CMR.
To our knowledge this is the first randomised trial to compare periprocedural injury following PCI and CABG using LGE CMR. Our study confirms the direct relationship between troponin elevation measured 24 hours after the procedure and the presence of LGE. The Synergy between PCI with Taxus and Cardiac Surgery (SYNTAX) trial recently reported no difference in death or myocardial infarction following CABG versus PCI for left main and/or triple vessel coronary artery disease10. Patients undergoing revascularisation in our study were representative of patients in the randomised Syntax trial with similar demography, disease severity and procedural outcomes to patients in the lower-middle tertile of the Syntax population.
In the Syntax trial, procedural myocardial infarction was part of the predefined primary endpoint. The definition used for events in the first seven days following revascularisation was a CK-MB/total CK ratio >10%, or CK-MB >5 x URL, for both groups, with the presence of new Q waves on ECG according to the Minnesota Code11. There was no infarct imaging in the protocol, and no imaging substudies. Troponin was not required for the definition of procedural myocardial infarction, and the specific question of procedure related necrosis and/or myocardial infarction was not examined. Similarly, other smaller studies of PCI with drug eluting stents for left main and/or triple vessel disease versus CABG have not reported the incidence of periprocedural injury12,13.
In our study, CMR defined injury following PCI was evident following clinically relevant complications (embolus or dissection with slow flow) and the overall incidence of CMR defined injury was relatively low for this complex patient subset (six out of 102 vessels). In previous studies we have shown a 28% incidence of CMR defined injury in complex PCI patients. In this current study was there was liberal use of IIb/IIIa inhibitors and fastidious technique to prevent side branch loss by pre-emptive wiring of branches and predominant use of second generation DES. These newer generation DES with thinner struts have been shown to reduce both side branch occlusion and the rates of non-Q wave myocardial infarction during revascularisation14.
We were not able to detect LGE reliably following PCI in patients with troponin elevation less than 4.0 ug/L. This may be a consequence of the multi-territory intervention that was performed which could reduce the ability of MRI to detect small areas of watershed ischaemia, and/or our inclusion of patients with pre-existing LGE. In all but one case of troponin elevation >4 ug/L we were able to detect new injury. The patient who underwent PCI with significant troponin I rise (5.6 ug/L) and no new LGE underwent rotational atherectomy, which may be relevant in the mechanism of injury. The majority of CABG patients with abnormal biomarker profiles and no new LGE had pre-existing infarction and it is possible that undetectable new injury may have occurred within these previously infarcted areas. Despite these factors, the direct relationship between troponin elevations measured 24 hours after the procedure and evidence of new LGE remains.
Applying the new definition of myocardial infarction to our sample resulted in a much higher incidence of myocardial infarction following PCI. Furthermore, the new definitions of PCI related necrosis and myocardial infarction are separated only by two times the URL of troponin, with necrosis defined as >3x URL and infarction defined as >5x URL. Accordingly, in our study, all of the patients with PCI related necrosis therefore also had PCI related myocardial infarction. Likewise, the new definition of CABG related necrosis appears to have questionable utility. In our study, all patients had troponin elevation following CABG, rendering 100% of patients with CABG related necrosis. Notably the definition of CABG related myocardial infarction differs from the PCI as it requires presence of new Q waves, angiographically documented graft or native vessel occlusion, and/or imaging evidence of myocardial infarction. Consequently, without routine CMR imaging in our study, the incidence of myocardial infarction after CABG would have been 0%. Interestingly, applying the surgical definition to our PCI population (adding the requirement of Q waves or imaging evidence of infarction) would result in the rate of MI falling from 30/40 (75.0%) to 6/40 (12.5%) in our cohort. These differences in definitions could result in confusion during future comparative revascularisation studies.
Study limitations
Our study was a single centre experience of PCI versus CABG, and thus caution should be applied when extrapolating these finding to different populations. The study is limited by its relatively small sample size and although there were more patients in the CABG group with CMR defined necrosis compared with PCI, this was not statistically different. Our study was only powered to detect a moderate difference in the frequency of periprocedural injury, and it is entirely possible that we have not detected a true, small difference in the rate of periprocedural injury between CABG and PCI. Furthermore, some patients with significant enzyme elevations were unable to undergo post-procedural CMR scans due to claustrophobia or impairment in renal function. Evolution of our local troponin assays has resulted in enhanced sensitivity of the test detecting a 10% imprecision at 0.05 ug/L, with a 99th percentile upper reference limit of 0.06 ug/L. This enhanced sensitivity of the troponin assay has direct impact on the rates of procedural necrosis as these definitions are relative to the upper reference limit.
Conclusions
We have shown that PCI can be performed without excess myocardial injury in patients with left main and/or triple vessel disease. The amount of injury as detected by new enzyme elevation with PCI appears to be less than with CABG, although this is not translated into a significant difference when assessed by LGE. However, when our results are viewed after application of the new definition of myocardial infarction, PCI fared worse than CABG, although the difference was entirely explained by inconsistencies with these definitions.
Acknowledgements
The authors are grateful to their colleagues, Drs Prendergast, Kharbanda, Alp, Choudhury, Cox, Kardos, Channon, Sprigings and Mulligan who allowed their patients to participate in the study.
Online trial registration:
ISRCTN25699844: http://www.controlled-trials.com/ISRCTN25699844

