Abstract
Background: Patients who are not candidates for traditional coronary artery bypass grafting (CABG) and amenable only for percutaneous coronary intervention (PCI) with stents can receive the “gold standard” left internal thoracic artery (LITA) to left anterior descending artery (LAD) anastomosis through robotic-assisted CABG and PCI to non-LAD coronary targets.
Aims: We aimed to analyse clinical outcomes of robotic-assisted CABG.
Methods: A total of 2,280 consecutive patients who had undergone robotic-assisted CABG between May 2005 and June 2021 were included in our study. Robotic-assisted LITA harvest was followed by LITA-LAD manual anastomosis through a 4 cm left thoracotomy. Hybrid coronary intervention (HCR) consists of stent implantation in a non-LAD coronary artery performed within 7 days after robotic-assisted LITA-LAD. We performed a propensity-adjusted analysis comparison after dividing all robotic-assisted CABG patients into three time periods: 2005-2010, 615 patients; 2011-2016, 904 patients; and 2017-2021, 761 patients.
Results: The mean age increased from 64.5 years in the first time period to 65.8 years in the second time period to 68.1 years in the third (p<0.0001). Operative time was progressively reduced in the three periods (6.4; 6.2; 5.5 hours; p<0.001). The incidence of conversion to sternotomy remained similar for each period (1.8%; 1.7%; 1.5%; p=0.53). Thirty-day mortality in the three periods included 9 (1.4%), 9 (1.0%), and 7 (0.9%) patients, respectively (p=0.91), while 8 (0.3%) patients had PCI with stents in the entire group. The mean follow-up for the entire population was 4.2 years. At follow-up, the rates of all-cause death, major adverse cardiac and cerebrovascular events, non-fatal stroke, and repeat revascularisation with stents were significantly decreased from the first to the last period (pË0.0001).
Conclusions: Robotic-assisted CABG and HCR provide good long-term outcomes in patients who are not candidates for conventional CABG.
Introduction
Robotic-assisted off-pump, coronary artery bypass grafting (CABG) consists of harvesting the left internal thoracic artery (LITA) followed by off-pump LITA to left anterior descending artery (LAD) anastomosis through a 4 cm left thoracotomy12. This procedure is an ideal approach for patients with multiple vessel disease, including LAD stenosis, and for patients who are not candidates for traditional sternotomy on-pump CABG but who are suitable for percutaneous coronary intervention (PCI)34. Through robotic-assisted CABG, patients can receive an ideal treatment combining the gold standard LITA anastomosis to the LAD and PCI treatment in other coronary vessels.
PCI treatment offers a very low risk of immediate complications and a more rapid recovery compared to saphenous vein grafts (SVG); the PREVENT IV (Prevention of Autogenous Vein Graft Failure in Coronary Artery Bypass Procedures) clinical trial reported an SVG failure rate of 45% at 12 to 18 months5. In addition, PCI relates primarily to the complexity of coronary artery disease, while the main benefits of CABG have been largely related to the LITA to LAD anastomosis.
LITA to LAD anastomosis followed by PCI treatment with drug-eluting stents (DES) has been named hybrid coronary revascularisation (HCR)6. In this context, HCR has been used mainly for elective surgical cases.
Adoption of robotic-assisted CABG has been low, with only 1% of the CABG procedures performed using a robot in North America7. The reason behind the low adoption rate of robotic-assisted CABG is multifactorial, including a low interest from surgeons, a steep learning curve, and lack of literature data to support the benefits of robotic-assisted CABG.
The two main goals of this manuscript are 1) to analyse the outcomes of our clinical experience in robotic-assisted CABG and 2) to report the outcomes of HCR, including repeat revascularisation, in this population.
Methods
PATIENT IDENTIFICATION AND STUDY POPULATION
We identified all consecutive patients who underwent robotic-assisted CABG between May 2005 and June 2021 at Lankenau Heart Institute (Lankenau Medical Center, PA, USA) and included them in the study. Patients were identified via operation codes in a digital operation registry, as well as from a centralised cardiac surgery database for all isolated robotic-assisted CABG operations. The study protocol was approved by the Main Line Health Hospitals Institutional Review Board on 11 November 2020 (IRB 45CFR164.512). Patients’ individual consent was waived due to the retrospective nature of the study.
PATIENT SELECTION PROCESS
Patients deemed suitable for robotic-assisted CABG had either isolated proximal LAD artery disease or multivessel disease in which the non-LAD artery disease was amenable to PCI with DES. Patients with multivessel disease referred for HCR strategy were discussed as part of a Heart Team approach with general and interventional cardiology and cardiac surgery. Indications for robotic-assisted CABG included 1) proximal LAD disease in patients for whom a stent for the LAD was considered not ideal due to young age or because the stent length would increase the risk of in-stent restenosis; 2) a chronic total obstruction (CTO) of the LAD, which can be associated with a second coronary artery disease lesion suitable for PCI and stenting; 3) patients with isolated left main (LM) coronary artery disease (CAD) and no (or minimal) artery disease in the other sites; this included patients for whom a conventional sternotomy CABG would have resulted in CABG with two grafts and in which the LAD would have received a LITA anastomosis and the circumflex artery would not be bypassed with a second arterial conduit; 4) patients with severe CAD and advanced age or comorbidities. The presence of multiple comorbidities combined with other factors increases the risk of perioperative complications and possibly mortality (Society of Thoracic Surgeons Predicted Risk of Mortality [STS-PROM] 3-10%). In these patients, a LITA-to-LAD bypass is feasible and recommended by the Heart Team, but complete surgical revascularisation with sternotomy and cardiopulmonary bypass (CPB) can be high risk. Complete revascularisation by PCI may not be feasible, and medical management may not be appropriate. The comorbidities may include, but are not limited to, any or a combination of the following: cerebrovascular disease, peripheral vascular disease (PVD), chronic kidney disease (CKD), osteoporosis, poorly controlled diabetes, advanced age, chronic anaemia, autoimmune disorders, and recent orthopaedic disorder; 5) patients awaiting transcatheter valve intervention; this included patients who were referred and accepted for transcatheter valve intervention but were found to have significant coronary artery disease. For these patients, a LITA-to-LAD bypass was recommended and feasible, but combined valve/CABG is at a very elevated risk (STS-PROM >8%); 6) patients with proximal LAD disease and CKD when a LITA-to-LAD bypass is strongly recommended by the Heart Team and feasible, and additionally where creatinine levels are chronically elevated but stable, there is no evidence of acute renal insufficiency, the STS-PROM is moderate (3-8%), and where patients are not suitable for either complete revascularisation by PCI or PCI to the LAD or LM coronary arteries; and 7) special circumstances in which patients declined CABG with sternotomy despite an appropriate conversation with the surgeon and interventional cardiologist on the benefits of CABG over PCI.
In our early experience, patients with a low STS-PROM risk score, favourable body habitus, and good sizeable coronary targets were considered for robotic-assisted CABG. As experience was gained, patients with a high risk score independent of body habitus, as well as those with calcified coronary arteries, were added as robotic-assisted CABG candidates.
THIRTY-DAY OUTCOMES AND PATIENT FOLLOW-UP
Thirty-day patient endpoint were collected in our database, including operative death, conversion to sternotomy, number of implanted stents, and graft closure necessitating treatment with PCI. Follow-up was done at our outpatient clinic and recorded in the hospital registry. All patients had at least one follow-up timepoint available. Patients who did not present for their clinical appointment were contacted by phone. If we were not able to contact the patient, we contacted the referring cardiologist to obtain the information needed for the study. In our centre, one surgeon performed CABG in the study timeframe (F.P. Sutter).
PRIMARY AND SECONDARY ENDPOINTS
The primary endpoint was an analysis of all-cause death for the designated three time periods after isolated robotic-assisted CABG. The secondary outcome was major adverse cardiac and cerebrovascular events (MACCE). In robotic-assisted CABG, HCR begins with LITA-to-LAD as the first step, followed by PCI with DES within 7 days. Therefore, the LAD is bypassed with a LITA-LAD anastomosis through a 4 cm left minithoracotomy, and the other vessels are treated with PCI within 7 days of the surgical procedure. All other variables were defined according to STS clinical guidelines.
STATISTICAL ANALYSIS FOR PROPENSITY MATCHING
Continuous variables were assessed for normality and are presented as means (standard deviation) or medians (interquartile range). Groups were compared by 2-sample t-tests or Wilcoxon rank-sum tests for continuous variables and the chi-square test of independence for categorical variables. A propensity-adjusted matching was used via a multiple logistic regression, with the first period (2005-2010) as the dependent variable and all demographics and preoperative variables added to the model. A 1:1 greedy nearest neighbour matching without replacement and a calliper width of 0.2 produced three groups, with the first group including 615 patients, the second group including 904 patients and third group including 761 patients. Success of matching was assessed by computing the percentage bias (similar to standardised mean difference) of each covariate, with a cut-off of 2% denoting acceptable balance. Matched samples were compared with McNemar’s test and marginal homogeneity tests for categorical variables and matched paired t-tests and signed-rank tests for continuous variables. Adjusted survival functions for these interactions were plotted using Stata’s st curve command. All analyses were performed in Stata 17.0 (StataCorp). We reported 95% confidence intervals and p-values, with a p-value<0.05 considered significant.
PROPENSITY ADJUSTMENT SIGNIFICANCE COMPARED TO PROPENSITY SCORE MATCHING
Propensity matching provides excellent matching before the analysis, while the propensity adjustment accounts for biases during the analysis. Therefore, when significant differences are seen between preoperative variables, these differences are adjusted during the modelling process. Propensity matching reduces the size of the groups, while propensity adjustment retains the sample size of the groups. As shown by multiple studies, propensity adjustments provide similar or better adjustments for biases when compared to propensity matching because in retaining the sample size, the statistical power of the analysis increases. This is particularly suitable for smaller sample sizes8.
COVARIATES AND EXPOSURES
Covariates included age, gender, race, STS-PROM score, body mass index (BMI), obesity, creatinine level, and comorbidities such as preoperative dialysis, smoking, chronic obstructive pulmonary disease, hypertension, dyslipidaemia, cerebrovascular disease, peripheral vascular disease, liver disease, diabetes, mediastinal radiation, prior PCI, prior CABG, prior myocardial infarction (MI), prior valve surgery, atrial fibrillation, ejection fraction, number of diseased vessels, left main coronary artery stenosis, severe proximal LAD lesion, and LITA and radial artery graft use.
P2Y12 BLOCKER MEDICAL THERAPY MANAGEMENT FOR HCR
Our preoperative medical management in patients undergoing HCR consists of robotic-assisted CABG performed on aspirin treatment, followed by P2Y12 blocker medical therapy on the day of PCI treatment.
SURGICAL TECHNIQUE
Robotic-assisted CABG includes the robotic harvest of the LITA followed by its direct anastomosis to the LAD with a small anterior thoracotomy (4 cm) at the site of the camera port (Moving image 1). The robotic procedure starts with the introduction of 3 ports in the midclavicular/anterior axillary line, in the 2nd, 4th and 6th intercostal spaces. A camera and two lateral arms with surgical instruments are introduced, and the surgeon sits at the robotic console while a tableside assistant positions the robotic surgical instruments (da Vinci Robotics; Intuitive Surgical). The three-dimensional view offered by the robotic platform enhances the visualisation of the LITA, lowering the risk of vessel injury and enabling the surgeon to harvest a longer LITA graft by means of skeletonisation. If a second ITA is required, the surgeon can open the right pleura crossing the mediastinum and access the right internal thoracic artery (RITA) from the left side of the chest. Our technique is based on “incision-precision”, where the camera port is located at the precise site of the LAD landing zone. By extending the camera port size to a 4 cm skin incision, the surgeon can perform manual off-pump coronary anastomosis using a composite off-pump retractor (Octopus Nuvo Tissue Stabilizer; Medtronic) and off-pump technique to complete the anastomosis. In cases where two arterial grafts are utilised, one side of the second arterial graft is anastomosed to the LITA (in a Y- or T-shaped anastomosis fashion) and the other side to the coronary targets (diagonal branch, intermediate branch, and obtuse marginal branch), either sequentially or end-to-end. If a vein graft is used, the proximal side is anastomosed to the LITA and the distal side to the other coronary targets.
Results
ALL ROBOTIC-ASSISTED CABG POPULATION
PREOPERATIVE CHARACTERISTICS
A total of 2,280 consecutive patients underwent robotic-assisted CABG at our institution between May 2005 and June 2021, which represents 46.7% of all 4,880 CABG cases over the same period. A total of 2,600 patients had conventional CABG during the same time period. A total of 615 patients were included in the first group (2005-2010), 904 patients were included in the second group (2011-2016) and 761 patients in the third group (2017-2021). The mean age increased from 64.5 years in the first time period to 65.8 years in the second time period and to 68.1 years in the third (p<0.0001). The percentage of females in the study decreased from 30.1% (n=185) in the first period to 27.4% (n=248) in the second period and to 23.3% (n=177) in the third one (p=0.015). The mean STS-PROM risk score decreased from 0.9% in the first period to 0.8% in the second period but went back up to 0.9% in the third period (p=0.008) (Table 1).
Table 1. Preoperative characteristics.
| Preoperative characteristics | 2005-2010 n=615 |
2011-2016 n=904 |
2017-2021 n=761 |
p-value |
|---|---|---|---|---|
| Age, years | 65.4±11.8 | 65.8±11.5 | 68.1±10.9 | <0.0001 |
| Female sex | 185 (30.1) | 248 (27.4) | 177 (23.3) | 0.015 |
| Race | 0.69 | |||
| White | 547 (88.9) | 808 (89.4) | 682 (89.6) | |
| Black or African American | 55 (8.9) | 76 (8.4) | 69 (9.1) | |
| Other | 13 (2.1) | 20 (2.2) | 10 (1.3) | |
| STS-PROM, % | 0.9 (0.4-2.3) | 0.8 (0.4-1.8) | 0.9 (0.5-2.0) | 0.008 |
| BMI, kg/m² | 29.2±5.4 | 29.4±6.1 | 29.0±5.2 | 0.445 |
| Obese >30 kg/m² | 224 (36.4) | 345 (38.2) | 286 (37.6) | 0.788 |
| Creatinine level | 1 (0.9-1.2) | 1 (0.8-1.2) | 1 (0.9-1.2) | 0.0002 |
| Chronic dialysis | 15 (2.4) | 18 (2.0) | 25 (3.3) | 0.243 |
| Smoking | 234 (38.1) | 346 (38.3) | 424 (55.7) | <0.0001 |
| COPD | 110 (17.9) | 132 (14.6) | 108 (14.2) | 0.121 |
| Hypertension | 517 (84.1) | 751 (83.1) | 663 (87.1) | 0.065 |
| Dyslipidaemia | 576 (93.7) | 852 (94.3) | 549 (72.1) | <0.0001 |
| CBVD | 69 (11.2) | 153 (16.9) | 176 (23.1) | <0.0001 |
| PVD | 92 (15.0) | 118 (13.1) | 92 (12.1) | 0.288 |
| Liver disease | 0 | 12 (1.3) | 19 (2.5) | <0.0001 |
| Diabetes | 223 (36.3) | 355 (39.3) | 313 (41.1) | 0.182 |
| Mediastinal radiation | 0 | 7 (0.8) | 22 (2.9) | <0.0001 |
| Previous PCI | 230 (37.4) | 429 (47.5) | 373 (49.0) | <0.0001 |
| Previous CABG | 7 (1.1) | 18 (2) | 5 (0.7) | 0.053 |
| Prior MI | 357 (58.1) | 523 (57.9) | 371 (48.8) | <0.0001 |
| Prior valve surgery | 0 (0) | 11 (1.2) | 11 (1.5) | 0.015 |
| Atrial fibrillation | 65 (10.6) | 111 (12.3) | 113 (14.9) | 0.054 |
| EF, % | 50.9±11.9 | 55.4±11.9 | 55.8±13.3 | <0.0001 |
| EF <50% | 165 (26.8) | 192 (21.2) | 173 (22.7) | 0.037 |
| Number of diseased vessels | 0.008 | |||
| 1 | 126 (20.5) | 158 (17.5) | 156 (20.5) | |
| 2 | 251 (40.8) | 356 (39.4) | 252 (33.1) | |
| 3+ | 238 (38.7) | 390 (43.1) | 353 (46.4) | |
| Left main coronary artery stenosis >50% | 79 (12.9) | 137 (15.2) | 126 (16.6) | 0.157 |
| Severe proximal LAD lesion >70% | 545 (88.6) | 819 (90.6) | 714 (93.8) | 0.003 |
| Data are reported as mean±SD, n (%) and median (IQR).P-values<0.05 are statistically significant. BMI: body mass index; CABG: coronary artery bypass grafting; CBVD: cerebrovascular disease; COPD: chronic obstructive pulmonary disease; EF: ejection fraction; IQR: interquartile range; LAD: left anterior descending artery; MI: myocardial infarction; PCI: percutaneous coronary intervention; PVD: peripheral vascular disease; SD: standard deviation; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality | ||||
INTRAOPERATIVE OUTCOMES
The number of bilateral ITA grafts decreased from 73 (11.9%) in the first period, to 14 (1.6%) in the second period and to 1 (0.1%) in the third period (Table 2). There was also a decrease in the number of total arterial (including 1 or more arterial grafts) and multiarterial (including more than 1 arterial graft plus veins) robotic-assisted CABG performed. Importantly, overall operative time decreased from 6.4 (±1.3) hours in the first period to 6.2 (±1.1) in the second period, then to 5.5 (±0.98) in the last period (Table 2, Table 3, Figure 1). In addition, overall blood products and red blood cell product transfusions significantly decreased during the respective time periods. Moreover, the rate of conversion to full sternotomy decreased from 1.8% (n=11) in the first period to 1.7% (n=16) in the second period, and then decreased to 1.5% (n=12) in the last period, although this did not reach statistical significance. HCR procedures with surgery followed by PCI with DES increased from 157 (25.5%) in the first period, to 343 (37.9%) in the second period up to 368 (48.4%) in the last period (p<0.0001).
Table 2. Intraoperative outcomes.
| Intraoperative outcomes | 2005-2010 n=615 |
2011-2016 n=904 |
2017-2021 n=761 |
p-value |
|---|---|---|---|---|
| ITA use | <0.0001 | |||
| Both ITA | 73 (11.9) | 14 (1.6) | 1 (0.1) | |
| Single or none | 542 (88.1) | 890 (98.5) | 760 (99.9) | |
| Radial artery graft use | 39 (6.3) | 56 (6.2) | 32 (4.2) | 0.131 |
| SVG | 54 (8.8) | 29 (3.2) | 23 (3.0) | <0.0001 |
| Number of grafts | 1.3±0.7 | 1.1±0.4 | 1.1±0.3 | <0.0001 |
| Number of grafts | <0.0001 | |||
| 1 | 474 (77.1) | 813 (89.9) | 704 (92.5) | |
| 2 | 103 (16.8) | 79 (8.7) | 52 (6.8) | |
| 3 | 24 (3.9) | 9 (1.0) | 3 (0.4) | |
| 4 | 11 (1.8) | 2 (0.2) | 2 (0.3) | |
| 5+ | 3 (0.5) | 1 (0.1) | 0 (0) | |
| Total arterial CABG | 103 (16.8) | 68 (7.5) | 32 (4.2) | <0.0001 |
| Multiarterial CABG | 335 (54.5) | 425 (47.0) | 297 (39.0) | <0.0001 |
| HCR procedures | 157 (25.5) | 343 (37.9) | 368 (48.4) | <0.0001 |
| HCR - number of planned stents implanted post-CABG | <0.0001 | |||
| 1 | 80 (13.0) | 173 (19.1) | 227 (29.8) | |
| 2 | 75 (12.2) | 150 (16.6) | 131 (17.2) | |
| 3 | 0 (0) | 10 (1.1) | 7 (0.9) | |
| Priority of surgery | 0.001 | |||
| Elective | 431 (70.1) | 589 (65.2) | 453 (59.5) | |
| Urgent | 183 (29.8) | 315 (34.9) | 306 (40.2) | |
| Emergent | 1 (0.2) | 0 | 2 (0.3) | |
| Time in OR, hours | 6.4±1.3 | 6.2±1.1 | 5.5±0.98 | <0.0001 |
| Overall blood products transfusions | 47 (7.6) | 54 (6.0) | 24 (3.2) | 0.001 |
| RBC units | 45 (7.3) | 51 (5.6) | 23 (3.0) | 0.001 |
| Cryoprecipitate units | 1 (0.2) | 6 (0.7) | 2 (0.3) | 0.241 |
| Platelet units | 4 (0.7) | 12 (1.3) | 4 (0.5) | 0.169 |
| FFP units | 1 (0.2) | 4 (0.4) | 2 (0.3) | 0.604 |
| Extubated in OR | 558 (90.7) | 774 (85.6) | 719 (94.5) | <0.0001 |
| Conversion to full sternotomy | 11 (1.8) | 16 (1.7) | 12 (1.5) | 0.53 |
| Data are reported as mean±SD or n (%). P-values<0.05 are statistically significant. CABG: coronary artery bypass grafting; FFP: fresh frozen plasma; HCR: hybrid coronary revascularisation; ITA: internal thoracic artery; OR: operating room; RBC: red blood cells; SD: standard deviation; SVG: saphenous venous grafts | ||||
Table 3. Postoperative outcomes.
| Postoperative outcomes | 2005-2010 n=615 | 2011-2016 n=904 | 2017-2021 n=761 | p-value | 1 vs 2 | 1 vs 3 | ||
|---|---|---|---|---|---|---|---|---|
| Adj. mean difference (95% CI) |
p-value | Adj. mean difference (95% CI) |
p-value | |||||
| Time in OR, hours | 6.4±1.3 | 6.2±1.1 | 5.5±0.98 | <0.0001 | –0.1 (–0.2 to 0.004 | 0.056 | –0.6 (–0.7 to –0.5) | <0.001 |
| Total time in ICU, hours | 26.5 (23.1-46.9) | 33.6 (24.1-69.6) | 29.6 (25.0-53.6) | 0.169 | 7.2 (–3.1 to 17.6) | 0.17 | –2.6 (–14.1 to 8.8) | 0.65 |
| Total LOS, days | 4 (4-6) | 4 (4-6) | 4 (3-5) | <0.0001 | –0.5 (–1.0 to –0.04) | 0.035 | –1.7 (–2.2 to –1.1) | <0.001 |
| Adj. odds ratio (95% CI) |
p-value | Adj. odds ratio (95% CI) |
p-value | |||||
| All types of blood transfusion products | 112 (18.2) | 144 (15.9) | 118 (15.5) | 0.357 | 0.9 (0.7 to 1.2) | 0.647 | 0.9 (0.7 to 1.3) | 0.575 |
| RBC units | 109 (17.7) | 139 (15.4) | 118 (15.5) | 0.417 | 0.9 (0.7 to 1.2) | 0.636 | 0.9 (0.7 to 1.3) | 0.799 |
| Cryoprecipitate units | 18 (2.9) | 22 (2.4) | 17 (2.2) | 0.706 | 0.8 (0.4 to 1.6) | 0.545 | 0.7 (0.4 to 1.6) | 0.431 |
| Platelet units | 28 (4.6) | 34 (3.8) | 11 (1.5) | 0.002 | 0.8 (0.5 to 1.4) | 0.433 | 0.3 (0.2 to 0.7) | 0.005 |
| FFP units | 11 (1.8) | 19 (2.1) | 9 (1.2) | 0.349 | 1.3 (0.6 to 2.8) | 0.551 | 0.7 (0.3 to 2.0) | 0.561 |
| Stroke | 2 (0.3) | 2 (0.2) | 6 (0.8) | 0.193 | 0.7 (0.1 to 5.0) | 0.689 | 1.6 (0.3 to 9.9) | 0.586 |
| Superficial wound infection | 0 (0) | 0 (0) | 0 (0) | NA | NA | NA | ||
| Reoperation for bleeding | 7 (1.1) | 8 (0.9) | 9 (1.2) | 0.814 | 0.8 (0.3 to 2.5) | 0.762 | 1.4 (0.5 to 4.4) | 0.516 |
| Prolonged ventilation >24 hours |
18 (2.9) | 15 (1.7) | 8 (1.1) | 0.031 | 0.6 (0.3 to 1.4) | 0.245 | 0.4 (0.1 to 0.9) | 0.04 |
| Renal failure | 13 (2.1) | 7 (0.8) | 2 (0.3) | 0.002 | 0.4 (0.1 to 0.9) | 0.041 | 0.1 (0.02 to 0.5) | 0.006 |
| New dialysis | 2 (0.3) | 2 (0.2) | 1 (0.1) | 0.747 | 1.2 (0.1 to 9.9) | 0.898 | 0.4 (0.02 to 7.3) | 0.516 |
| Postoperative atrial fibrillation | 94 (15.3) | 162 (17.9) | 126 (16.6) | 0.396 | 1.2 (0.9 to 1.6) | 0.148 | 1.0 (0.8 to 1.4) | 0.776 |
| 30-day readmission | 71 (11.5) | 85 (9.4) | 67 (8.8) | 0.208 | 0.8 (0.6 to 1.1) | 0.192 | 0.7 (0.5 to 1.0) | 0.057 |
| 30-day all-cause mortality | 9 (1.5) | 9 (1.0) | 7 (0.9) | 0.586 | 0.8 (0.3 to 2.2) | 0.654 | 0.9 (0.3 to 2.9) | 0.918 |
| Data are reported as mean±SD, n (%) and median (IQR). P-values<0.05 are statistically significant. Adj.: adjusted; CI: confidence interval; FFP: fresh frozen plasma; ICU: intensive care unit; IQR: interquartile range; LOS: length of stay; NA: not available; OR: operating room; RBC: red blood cells; SD: standard deviation | ||||||||
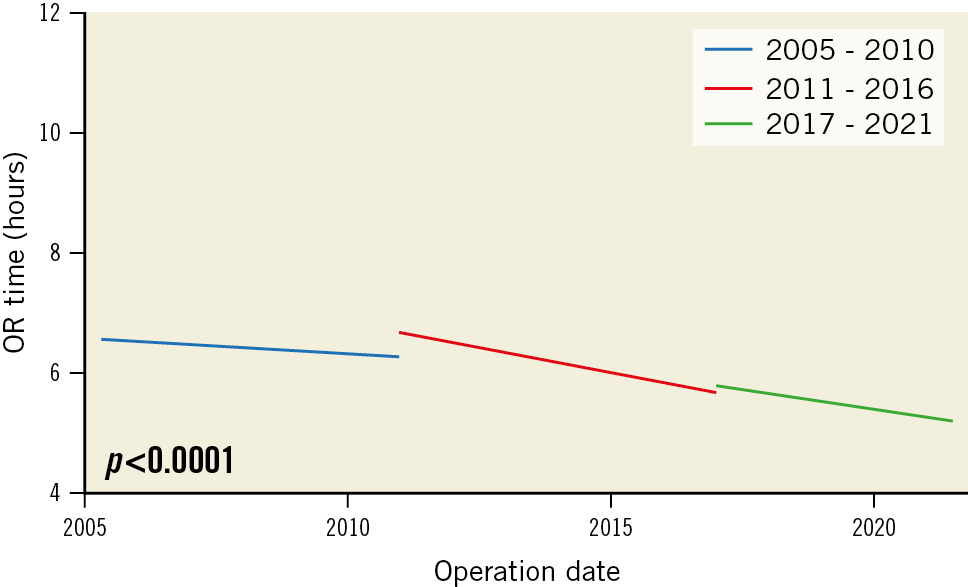
Figure 1. Evolution of OR time over the three time periods. OR: operating room
POSTOPERATIVE OUTCOMES
The total hospital length of stay (LOS) (p<0.001), platelet unit transfusion products (p=0.005), use of prolonged ventilation (>24 hours) (p=0.006) and the incidence of postoperative renal failure (p=0.006) all significantly decreased in the last period when compared to the first and second periods (Table 3). One patient, who went to the operating room (OR) for postoperative bleeding, had to be converted to sternotomy. At 30 days, no patient had undergone a repeat surgical intervention. In addition, 7 (0.8%) patients who underwent HCR had PCI with one DES on the LITA-to-LAD anastomosis (due to graft failure), and 1 (0.1%) patient that underwent HCR had two DES implanted, one on the LITA-to-LAD and another on a radial artery graft on a diagonal coronary artery (the graft failure of LITA and the radial arteries were in the same patient).
FOLLOW-UP OUTCOMES
The mean follow-up for each time period was 3.5 years. At follow-up, the incidence of all-cause death decreased from 30 (4.9%) in the first period to 29 (3.8%) in the last period (p<0.0001) (Table 4, Figure 2, Supplementary Figure 1). In the first year, 2 (0.3%), 3 (0.3%), and 2 (0.2%) patients died, respectively. By the fifth year, an additional 28, 41, and 26 patients experienced all-cause mortality. MACCE incidence decreased from 158 (25.7%) in the first period to 125 (16.6%) in the last period (p=0.0002). In addition, stroke incidence decreased from 16 (2.6%) in the first period to 4 (0.5%) in the last period (p<0.0001). The incidence of MI increased from 11 (1.8%) in the first period to 18 (2.4%) in the last period (p=0.04). Repeat revascularisation with stents decreased from 99 (16.3%) in the first period to 74 (9.8%) in the last period (p<0.0001). At follow-up (Table 5, Supplementary Figure 2), 139 HCR patients had undergone repeat revascularisation with PCI. A total of 102/139 (73.4%) patients had at least one non-previous surgical target vessel treated by PCI with DES. The incidence of PCI on the LITA-LAD surgical target vessel was 14.4% (20 patients), PCI of an occluded in-stent LAD was 2.2% (3 patients) and PCI on LAD for new disease/stenosis was 7.2% (18 patients) (Table 5). In addition, 26 (18.7%) patients had PCI on the right coronary artery (RCA) for an in-stent occlusion, while 51 (36.7%) patients had PCI on the RCA for new disease/stenosis. For patients in whom the diagonal coronary artery was treated, 4 (2.9%) patients had a surgical bypass on the vessel, while 1 (0.7%) patient had PCI for in-stent stenosis, and 6 (4.3%) patients had PCI for new disease/stenosis. Finally, 1 (0.7%) patient had PCI in an obtuse marginal/ramus artery that was a previous surgical target, 19 (13.7%) patients had PCI on the obtuse marginal/ramus arterty for in-stent stenosis, and 53 (38.1%) for new disease/stenosis.
Table 4. All population follow-up outcomes.
| Follow-up outcomes | 2005-2010 N=606 |
2011-2016 N=895 |
2017-2021 N=754 |
p-value |
|---|---|---|---|---|
| All-cause death | 30 (4.9) | 44 (4.9) | 29 (3.8) | <0.0001 |
| MACCE | 158 (26) | 236 (26.3) | 125 (16.6) | 0.0002 |
| Stroke | 16 (2.6) | 14 (1.5) | 4 (0.5) | <0.0001 |
| MI | 11 (1.8) | 35 (3.9) | 18 (2.4) | 0.04 |
| Repeat revascularisation with stents | 99 (16.3) | 143 (16) | 74 (9.8) | <0.0001 |
| Repeat revascularisation with CABG | 1 (0.2) | 0 | 0 | 0.6 |
| Data are reported as n (%). P-values <0.05 are statistically significant. CABG: coronary artery bypass grafting; MACCE: major adverse cardiac and cerebrovascular events; MI: myocardial infarction | ||||
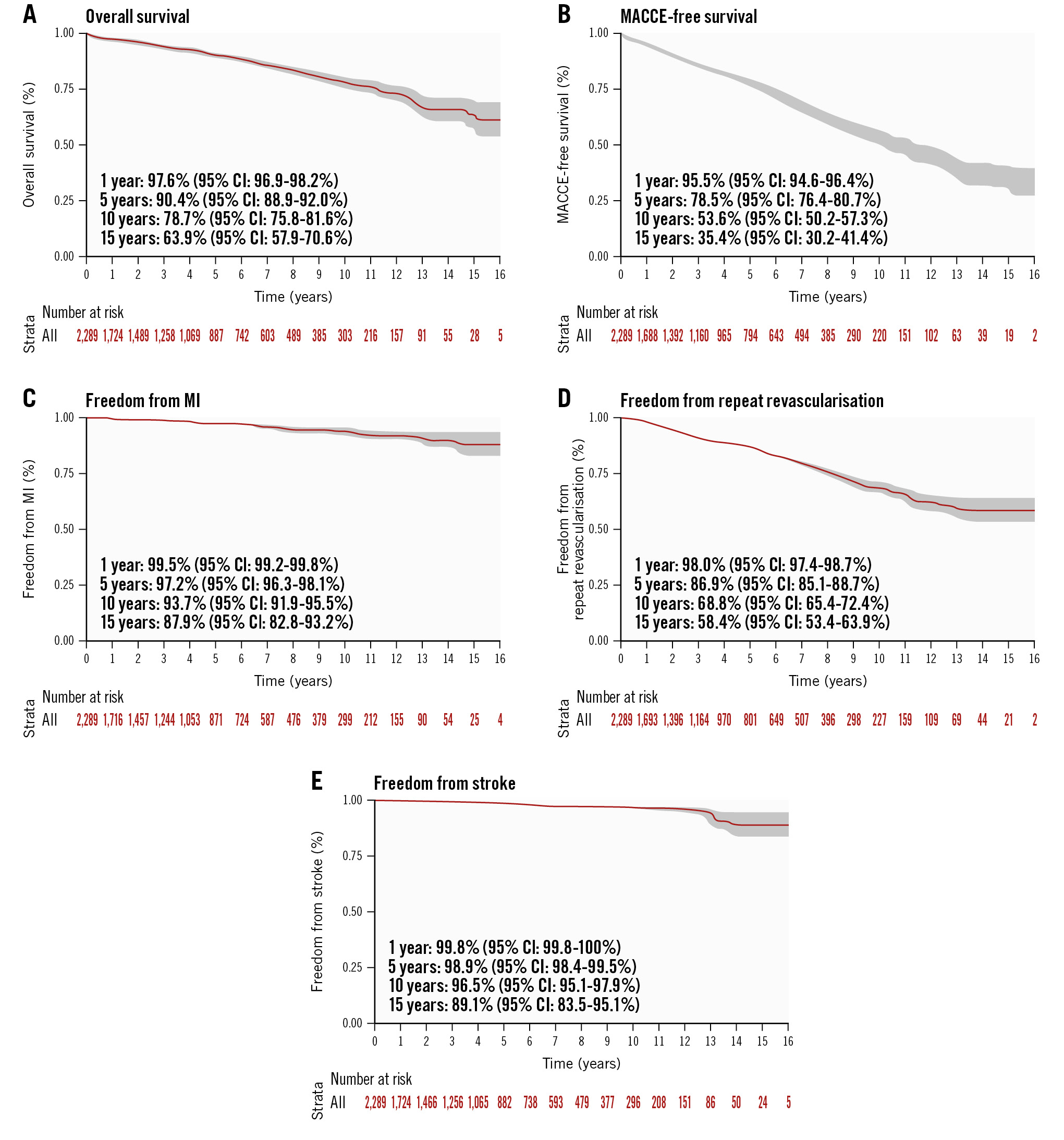
Figure 2. Kaplan-Meier curves for the entire study population (2005-2021). CI: confidence interval; MACCE: major adverse cardiac and cerebrovascular events; MI: myocardial infarction
Table 5. Repeat revascularisation in HCR population.
| Repeat revascularisation at 15-year follow-up | N=139 |
|---|---|
| Repeat intervention with PCI | 139 (100) |
| Repeat surgical interventions | 0 (0) |
| Patients with at least 1 new vessel disease | 102 (73.4) |
| Repeat PCI on LITA-LAD surgical target (graft failure) | 20 (14.4) |
| Repeat PCI on LAD occluded in-stent | 3 (2.2) |
| Repeat PCI on RCA for in-stent occlusion | 26 (18.7) |
| Repeat PCI on previous diagonal surgical target (graft failure) | 4 (2.9) |
| Repeat PCI on diagonal for in-stent stenosis | 1 (0.7) |
| Repeat PCI on ramus/OM previous surgical target (graft failure) | 1 (0.7) |
| Repeat PCI on ramus/OM for in-stent stenosis | 19 (13.7) |
| New PCI on new LAD for new disease/stenosis | 18 (7.2) |
| New PCI on RCA for new disease/stenosis | 51 (36.7) |
| New PCI on diagonal for new disease/stenosis | 6 (4.3) |
| New PCI on ramus/OM new disease/stenosis | 53 (38.1) |
| Data are reported as n (%). HCR: hybrid coronary revascularisation; LAD: left anterior descending; LITA: left internal thoracic artery; OM: obtuse marginal; PCI: percutaneous coronary intervention; RCA: right coronary artery | |
Discussion
ADDRESSING THE LITERATURE DATA GAP
The evolution of robotic-assisted CABG has progressed slowly, mainly due to a lack of published data and a steep learning curve (Central illustration). To address the learning curve issue, Whellan and colleagues7 described the 10-year follow-up outcomes and the learning curve after the treatment of 1,000 patients. The authors reported an overall operative mortality of 6 patients, 30 patients were converted to sternotomy, 10 patients required a 30-day repeat intervention, and 108 patients were transfused postoperatively. The analyses showed that the mastery of robotic-assisted CABG can be achieved after performing between 250-500 surgical cases. Therefore, a minimum of two years is required to master this technology. Our centre is visited every year by clinical fellows and novel surgeons who come to master the robotic-assisted CABG procedure. The training that these surgeons receive allows them to bring the robotic CABG procedure to their practice; surgeons who now practise robotic-assisted CABG procedures after having trained in our centre include surgeons in New York, Europe, Saudi Arabia, Japan, etc. In 2022, a new surgeon (G. Torregrossa) joined our clinical practice, and in July of this year, another surgeon (D. Spragan) joined us, therefore, making Lankenau an even busier clinical centre performing robotic-assisted CABG.
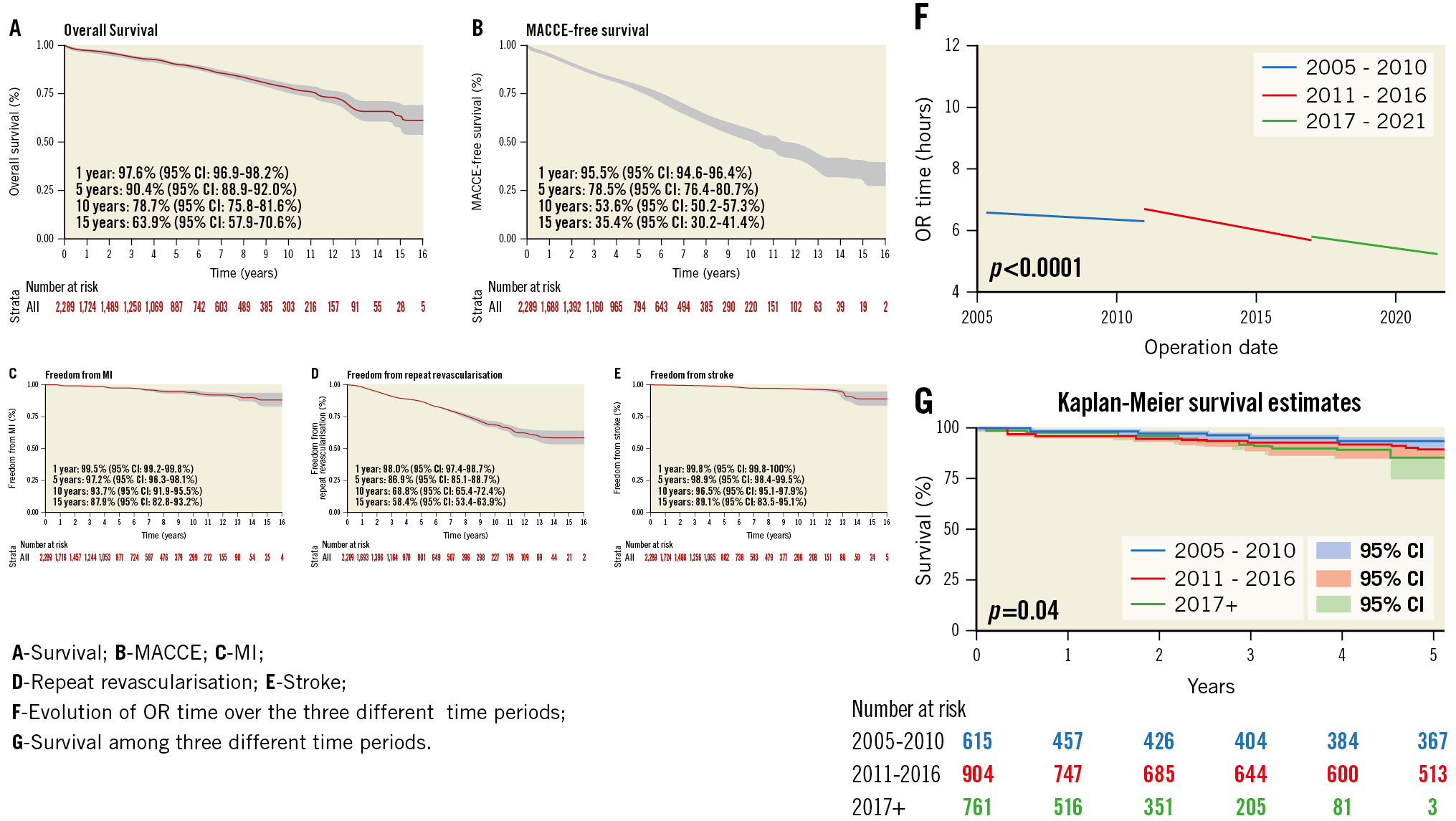
Central illustration. Kaplan-Meier curves. CI: confidence interval; MACCE: major adverse cardiac and cerebrovascular events; MI: myocardial infarction; OR: operating room
OPERATIVE TIME
Overall OR time for robotic-assisted CABG is longer when compared to conventional CABG. In this context, the ROOBY clinical trial reported a mean OR time of 4.5±1.4 hours for off-pump CABG patients and 4.4±1.3 hours for on-pump patients, while our study reported a mean OR time of 6.4 hours in the first period which decreased to 5.5 hours in the third period. However, the operative time in this study is similar to the one published by Whellan et al7. In this context, we must note that operative time is not only related to the surgeon but also to the experience gained from the entire Heart Team, including anaesthetists, nurses, clinical assistants, etc. Therefore, OR time reflects the cumulative time of the entire cardiac team. Technological progression has been aided by advancements in technology since 2005, therefore, decreasing the amount of time necessary for specific portions of the surgical procedures. In addition, training clinical fellows in robotic-assisted CABG, as done in our centre, further increases operative time.
CLINICAL OUTCOMES COMPARED TO CLINICAL TRIALS
Five-year outcomes from the ROOBY clinical trial showed patients undergoing off-pump CABG had a conversion rate to on-pump CABG of 12.4%, use of blood transfusion products in 52% of cases, a postoperative atrial fibrillation (POAF) incidence of 49.7%, a repeat revascularisation rate of 13.1% and hospital LOS of 8.2 (±8.8) days9. In addition, the five-year outcomes from the CORONARY clinical trial for patients undergoing off-pump CABG evidenced a rate of conversion to on-pump CABG of 7.2%, utilisation of blood transfusion products in 50.7% of patients, a POAF incidence of 18.3%, a repeat revascularisation incidence of 2.8%, and hospital LOS of 8 days10. The FREEDOM clinical trial revealed a staggering 85% survival rate at 8-year follow-up11. However, the mean age of the study population was 8 years younger than the one of this study. When compared to postoperative outcomes from the three aforementioned clinical trials, this analysis evidenced similar or better outcomes. Hospital LOS is another major predictor of clinical outcomes after CABG12. In this context, LOS is impacted by many factors, including new POAF incidence, which is known to be associated with an increased incidence of stroke, MACCE, heart failure, and overall mortality13. Factors related to an increased incidence of POAF after CABG include heart manipulation during surgery, potassium and calcium imbalance, and the use of CPB14. Robotic-assisted CABG has minimal heart manipulation, while most procedures were performed off-pump, reducing the incidence of POAF and, therefore, decreasing hospital LOS. In this context, our mean LOS was 4 days while the ROOBY clinical trial had a hospital LOS of 8.2 (±8.8) days, and the CORONARY trial had a mean LOS of 8 days. Predictors of LOS include repeat CABG, chronic heart failure, renal failure, and diabetes mellitus12. In our study, all these predictors are similar to those described in the ROOBY and CORONARY clinical trials.
HYBRID CORONARY REVASCULARISATION
HCR combines the best of the two worlds by providing patients with the “gold standard’’ LITA to LAD anastomosis followed by PCI with stent to non-LAD vessels in candidates not amenable to traditional CABG but only to PCI with stents.
In this analysis, we provided granular 30-day and long-term follow-up clinical outcomes of patients undergoing HCR. In addition, this analysis provides novel insights on the feasibility of HCR, therefore, answering critical questions on the presence of postoperative bleeding, amount of blood product transfusion rates, operative death, and repeat coronary intervention. A previous 30-day outcome report of 106 patients from Repossini and colleagues15 evidenced a transfusion rate of 30%, intensive care unit stay of 20 hours and hospital LOS of 6.4 days. However, data granularity on specific target repeat revascularisation outcomes was missing. In this context, this analysis provided new data on surgical revascularisation targets at long-term follow-up.
HYBRID TEAM
The development and maintenance of a coronary team and close collaboration among surgeons are crucial to the achievement of good results. In this regard, a close collaboration among cardiologists and cardiac surgeons, where both PCI with DES and CABG play a crucial role, is a condition sine qua non for the continuum of care.
INCIDENCE OF REPEAT REVASCULARISATION
At five-year follow-up, the NOBLE clinical trial reported an overall incidence of surgical target lesion repeat revascularisation of 10%16. At 3-year follow-up, the CORONARY clinical trial evidenced an overall incidence of repeat revascularisation of target vessels of 7.1%. The SYNTAX clinical trial reported an overall incidence of 18% of repeat revascularisation at 5-year follow-up, whereas, our overall incidence of repeat revascularisation of all patients from 2005-2021 was 13%, which is lower than the five-year clinical outcomes from the SYNTAX clinical trial. In addition, the CORONARY and the NOBLE trials outcomes were only reported at 3- and 5-year follow-up, respectively. Therefore, the outcomes from our study are in line with those of other clinical trials and demonstrate non-inferiority of clinical outcomes for robotic-assisted CABG compared to conventional CABG.
STRENGTHS OF THE PROCEDURE
Robotic-assisted CABG offers a valid alternative to PCI in patients who are not candidates for conventional CABG. The procedure has a steep learning curve, and it takes a minimum of two years to be mastered by surgeons. However, it provides patients with an alternative to PCI with drug-eluting stent (DES), increases overall referrals from cardiologists, increases the practice volume, and provides a real-world effective Heart Team collaboration among cardiologists, cardiac surgeons, and interventional cardiologists.
Limitations
The main limitations from this study include the lack of a SYNTAX score, the long timespan and being a single-surgeon, single-institution experience. A recently published paper from Emory University, also with the limitations of a single-centre study, reports good results1718.
Conclusions
This single-centre study reports very long-term follow-up clinical results of robotic-assisted CABG procedures. Our results provide good long-term outcomes in patients who are not candidates for conventional cardiac surgery.
Impact on daily practice
Patients who are not candidates for traditional cardiac surgery, but are candidates for PCI, can benefit from robotic-assisted CABG with the “gold standard LITA-LAD’’ followed by PCI to other non-LAD vessels.
Data availability statement
The data that support the findings of this study are available upon reasonable request to Dr Serge Sicouri, pending institutional approval.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Robotic-assisted coronary artery bypass grafting technique.

