Introduction
Transcatheter aortic valve implantation (TAVI) has become a therapeutic standard for patients with symptomatic severe aortic stenosis (AS) across all surgical risk categories. In the European guidelines for the management of patients with valvular heart disease, TAVI received a class 1 recommendation for the treatment of patients with symptomatic severe AS aged ≥75 years, and the lower age limit was set as low as 65 years in the American guidelines12. Both the European and American guidelines emphasise the importance of balancing the life expectancy of the patient with the valve durability when considering a surgical or transcatheter bioprosthetic valve. However, robust data on long-term transcatheter heart valve (THV) durability are scarce, as TAVI remains a relatively new treatment modality, and initially, only high-risk patients with limited life expectancy were treated with TAVI.
To what extent the currently treated TAVI populations, both in clinical trials and in daily clinical practice, will allow us to collect reliable large-scale data on THV durability exceeding 10 years remains unknown. Considering the advanced age, comorbidities and frailty of patients treated with TAVI over the past decade, this population may have too low of a survival rate to reliably study long-term THV durability. Today, the only randomised TAVI vs SAVR trial with more than half of its initial study population still alive at 8 years of follow-up is the NOTION trial, which was conducted from 2009 to 20133.
The aim of this analysis was to explore long-term survival in an all-comers TAVI population and to identify which patient category could provide long-term durability data on transcatheter bioprosthetic aortic valves.
Methods
We retrospectively analysed all patients undergoing TAVI in East Denmark between 2008 and 2021, a dataset with 100% complete survival follow-up. The patients were classified as low (<4%), intermediate (4-8%) or high surgical risk (>8%), based on EuroSCORE II. Using Cox proportional regression analysis, we identified predictors of survival and compared the survival rates of different populations.
Results
In total, 2,670 patients (mean age 79±7 years, 58% male) underwent TAVI, predominantly with self-expanding THV (89.6%) (Figure 1A). The proportion of patients with a bicuspid aortic valve was noticeably larger in younger patients (Figure 1B). The overall survival in the entire TAVI population was 58% at 5 years and 20% at 10 years (Figure 1C).
In a Cox proportional regression analysis, we identified age at the time of the procedure (hazard ratio [HR] 1.11; p=0.001), surgical risk category (HR 1.29; p<0.001), and the treatment period (HR 1.13; p=0.035) as independent predictors of overall mortality (Figure 1D). We then compared survival according to age and risk categories. The 10-year survival rates for patients <75 years of age were better compared to those aged 75 years and older (Figure 1E). These younger patients, especially those treated in the most recent treatment period, had better survival, while there was no difference according to the calculated surgical risk (Figure 1F).
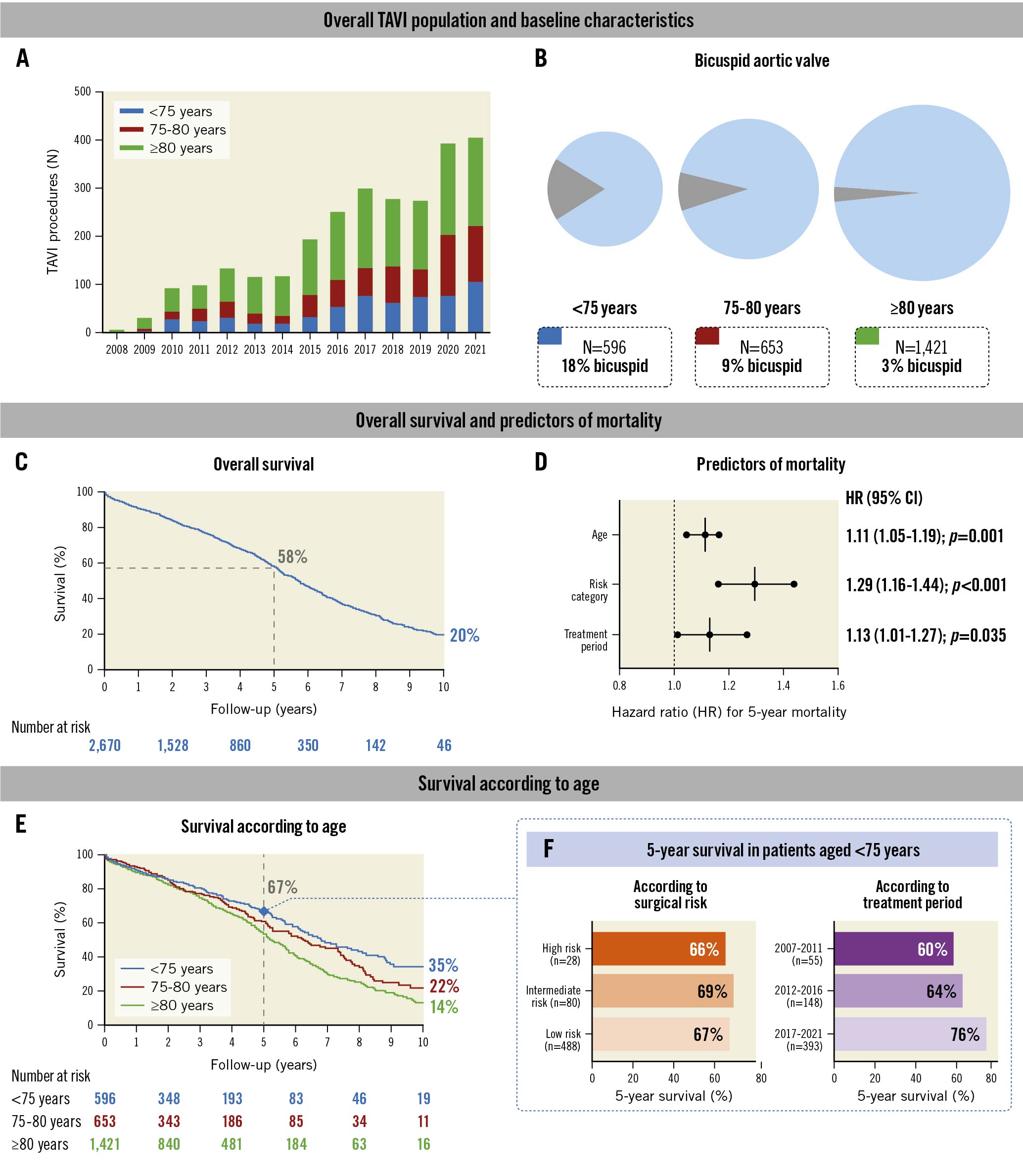
Figure 1. Identifying TAVI patients that could provide long-term valve durability data. Data on TAVI in younger patients <75 years of age with a low burden of comorbidities, not excluding bicuspid aortic valves, will be needed to obtain long-term THV durability in the future. Predictors of mortality in (D) were age (per 5-year increase), surgical risk (per increase in risk category) and treatment period (per 5-year decrement, for the periods 2017-2021, 2012-2016 and 2007-2011). CI: confidence interval; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve
Discussion
We herein show that the 10-year survival rate of patients aged 75 years and over is low. Their survival is not any different from a background population with a similarly high age4. This implies that European registry-based data will not generate the long-awaited, long-term THV durability data, as the European guidelines do not recommend routine TAVI in patients aged <75 years. The 5-year survival rate of patients <75 years of age and treated in the more recent 2017-2021 period was markedly better, i.e., 76%. Therefore, to obtain a large enough patient cohort still alive 10 years after TAVI, we will need to enrol younger patients, treated with contemporary techniques. In the hypothetical scenario that structural valve deterioration at 10 years would be 10% and 20% following TAVI and SAVR, respectively3, 800-1,000 patients younger than 75 years should be enrolled in order to have 400 study subjects alive at 10 years and, eventually, be able to demonstrate a statistically significant difference in valve durability between both modalities.
It is reassuring to note that survival rates of the younger TAVI patients treated in the 2017-2021 period were markedly higher compared to the younger patients treated with TAVI in the earlier periods. This can be explained by the fact that only younger patients with multiple comorbidities, which do not necessarily translate into a high calculated surgical risk score, were treated with TAVI in the early days. Calculated surgical risk scores are indeed largely dependent on the patient’s age.
As younger patients, aged 65 to 75 years, can nowadays be offered TAVI in the USA, this cohort of younger TAVI patients should be followed with increased interest. Ultimately, there will be a need for robust randomised controlled trials comparing long-term valve durability following TAVI and SAVR in patients aged <75 years, including patients with bicuspid AS.
Limitations
Despite the 100% complete follow-up of all consecutive TAVI patients, the current exploratory analysis is limited by the short follow-up period of the most recently treated patients. Moreover, a limited number of variables were considered in the regression model, as we focused on age, the calculated surgical risk and treatment periods, reflecting contemporary practice, and patient selection.
Conclusions
In conclusion, it will require a dedicated randomised clinical TAVI vs SAVR trial, including only younger patients less than 75 years of age with a low burden of comorbidities – not excluding bicuspid AS – to shine a light on long-term THV durability in the future (Figure 1).
Conflict of interest statement
The authors have no conflicts of interest to declare.

