Abstract
Aims: Increased major adverse cardiac events (MACE) beyond six months after intracoronary β radiation brachytherapy (IRBT) are a major concern. The aim of this study was to evaluate the 10-year clinical outcome after IRBT.
Methods and results: From 1997 to 2002, 301 consecutive patients treated with IRBT were included prospectively, whereafter 602 control patients treated with conventional percutaneous coronary intervention (PCI) were matched by propensity score methodology. MACE was defined as all-cause death, any myocardial infarction or any revascularisation. Median follow-up duration was 9.7 years. Mortality rates in both groups were similar. Cumulative 5-month, 2-, and 10- year MACE-free survival rates of IRBT patients were 89%, 56% and 29%, respectively, while those of the control patients were 90%, 76% and 52%, respectively (p<0.001). The difference in the MACE rate was mainly driven by target vessel revascularisation (TVR) (p<0.001). Furthermore, two or more repeat TVRs were needed in 12% of IRBT patients and in only 6% of control patients (p<0.01). Adjusted hazard ratios for IRBT-associated all-cause mortality and MACE were 1.0 (95% CI 0.7-1.5) and 1.8 (95% CI 1.5-2.2), respectively.
Conclusion: IRBT was associated with increased MACE between five months and two years of follow-up, mainly driven by repeat revascularisations. Similar event rate after two years indicate that there were no very late adverse effects related to IRBT.
Introduction
Restenosis caused by neointimal tissue proliferation has been a major limitation to long-term success of percutaneous coronary intervention (PCI).1 Prior to the availability of drug-eluting stents, intracoronary radiation brachytherapy (IRBT) has been evaluated as a treatment for prevention of restenosis making use of its strong antiproliferative effect. Randomised studies have initially shown safety and efficacy of IRBT for treatment of in-stent restenosis using β and γ emitters delivered by catheter-based systems.2-7 However, effectiveness in de novo lesions is questionable.8-12 Furthermore, restenosis observed at the edges of the irradiated lesions, the so-called edge effect, was a major concern in the beginning of the brachytherapy era.10,13 This resulted in increased rates of adverse cardiac events beyond the first six months after IRBT treatment.14
By the year 2000 IRBT had become an established technique, and is still recommended as an adjunctive treatment for in-stent restenosis by the latest guidelines.15,16 The risk of the edge effect was minimised by the use of long sources (or sequential pull-back technique) that effectively irradiate the complete vessel segment of interest.17,18 Several studies have reported maintained overall clinical benefit of IRBT for in-stent restenosis during a follow-up period of up to five years.19-23
The aim of this study was to evaluate late clinical outcome and potential very late adverse effects after intracoronary β radiation brachytherapy in comparison to a matched control group treated with standard routine PCI. In addition, clinical outcomes were evaluated separately in predefined subgroups (specifically, IRBT for de novo lesions and IRBT for restenosis). These long-term results might improve our understanding on the potentially long lasting interaction of localised β radiation therapy on vascular response in humans.
Methods
Patient inclusion and data collection
Between April 1997 and December 2002, all consecutive patients (n=301) treated with intracoronary β radiation brachytherapy adjunctive to PCI at Erasmus University Medical Center in Rotterdam, The Netherlands, were included prospectively. Five-year outcomes of these IRBT patients were reported previously.14 To evaluate IRBT related outcomes, we compared the IRBT patients with control patients who were treated with standard routine PCI during the same inclusion period. A total of 5,224 patients were treated with standard routine PCI in our institution. For the acquirement of a representative control group, propensity score methodology was used to match the 301 IRBT patients with 602 control patients. In addition, we performed sub-analyses in the following predefined subgroups: IRBT for de novo lesions and IRBT for restenosis. Baseline and procedural characteristics were acquired prospectively by recording the data in a dedicated database.
Study endpoints and definitions
Primary endpoints were: all-cause mortality and the patient oriented composite endpoint of major adverse cardiac events (MACE) according to ARC definitions,24 which included all-cause mortality, any myocardial infarction (MI) and any repeat revascularisation. Secondary endpoints were: any MI, the composite endpoint of all-cause mortality or any MI, repeat revascularisation, target vessel revascularisation (TVR), and second repeat target vessel revascularisation. MI was diagnosed by an increase in creatine kinase-MB fraction of three times the upper limit of normal, together with electrocardiographic changes and symptoms suggestive of ischaemia.25 Repeat revascularisation was defined as any PCI or CABG during follow-up. TVR was defined as any repeat revascularisation of any segment of the target vessel. Primary study outcomes were evaluated at five months, 2-, and 10- years of follow-up. For the evaluation of IRBT efficacy, the follow-up period of
five months was a priori chosen, so that potential repeat revascularisations performed during angiographic follow-up at six months after IRBT could be excluded.
Follow-up
In January 2009, vital status of all patients was acquired from municipal civil registries. Questionnaires were sent to all living patients focusing on the occurrence of MACE. The referring physician and institutions as well as the general practitioners were directly approached whenever necessary.
Statistical analysis
Propensity score methodology was used to identify comparable patients who were treated with standard routine PCI. The propensity score was initially proposed by Rosenbaum and Rubin and has been used in prior observational studies to help adjust for treatment selection bias.26-28 First, a propensity score for each patient was constructed, providing an estimate of the propensity toward belonging to one treatment group versus the other. This was done by using a multivariable logistic regression model with the type of intervention (standard routine treatment coded as 0, IRBT as 1) as the dependent variable. The following variables were entered into the model as independent variables: age, gender, diabetes mellitus, hypertension, smoking, prior MI, prior CABG, prior PCI, multivessel disease, impaired left ventricular function (ejection fraction <40%), and clinical presentation. Second, each IRBT patient was matched with two control patients with identical propensity score by four decimals.
All data were analysed with SPSS software (SPSS 15.0; SPSS Inc., Chicago, IL, USA). Continuous variables were compared by Student t test and are presented as mean ±standard deviation. Categorical variables were compared by chi-square test or Fisher’s exact test, when appropriate, and are presented in percentages. Patients lost to follow-up were considered at risk until the date of last contact, at which time point they were censored. Cumulative (event-free) survival was estimated according to the Kaplan-Meier method. Kaplan-Meier survival curves were compared by log-rank test. Univariate and multivariate Cox regression analyses were performed to evaluate IRBT associated outcomes. In multivariate analyses, the following variables were entered into the model: IRBT treatment, age, gender, diabetes mellitus, hypertension, smoking, prior MI, prior CABG, prior PCI, multivessel disease, impaired left ventricular function (EF<40%), clinical presentation and indication for PCI (de novo lesion/restenosis). The final results are presented as unadjusted and adjusted hazard ratios (HR), with the associated 95% confidence intervals (95% CI). All statistical tests were two-tailed, where a p-value <0.05 was considered statistically significant.
Results
Baseline characteristics
Baseline characteristics of IRBT patients (n=301) and control patients (n=602) are presented in Table 1.
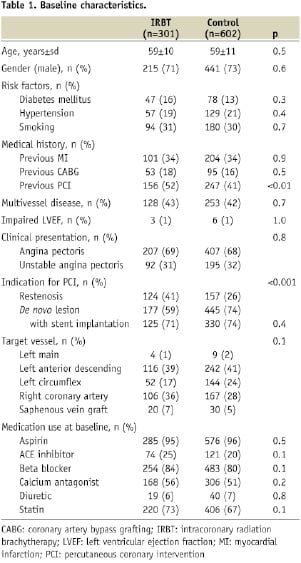
Most of the baseline characteristics were similar in the two groups, although the IRBT group had more patients with a history of previous PCI (IRBT 52% versus control 41%, p<0.01) and more patients treated for restenosis (IRBT 41% versus control 26%, p<0.001). Radiation therapy details are presented in Table 2.

Most of the IRBT patients were originally included in several randomised controlled trials or registries.6,9-11,18,29,30 Strontium-90/yttrium-90 source was used in 87%, and phosphorus-32 source was used in 13% of IRBT patients.
Long-term outcome
Clinical follow-up was available for 96% of the patients. Response rate of the questionnaires that were sent to all living patients was 94%. Median follow-up duration was 9.7 years and ranged from six to 12 years.
The incidence of the study endpoints at 10 years as well as the unadjusted and adjusted hazard ratios are presented in Table 3.
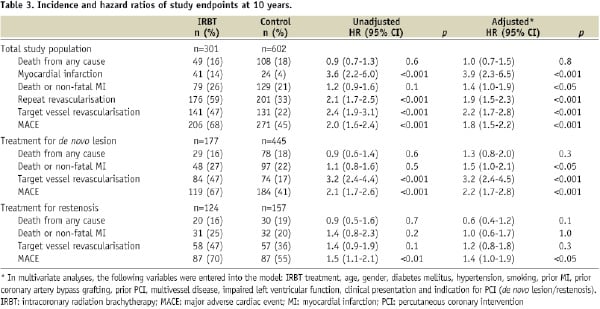
There were a total of 49 deaths (16%) in the IRBT group and 108 deaths (18%) in the control group. The Kaplan-Meier cumulative survival curves are displayed in Figure 1A. Cumulative 1-, 5-, and 10- year survival rates were 98%, 90% and 82% in IRBT patients, respectively, and were 98%, 92% and 79% in the control patients, respectively (log rank p=0.6). After adjustment in multivariate analysis, the adjusted hazard ratio for IRBT associated all-cause mortality was HR 1.0, 95% CI 0.7-1.5.
Although mortality was similar, more MACE was observed in the IRBT group than the control group (IRBT 68% versus control 45%). The Kaplan-Meier cumulative MACE-free survival curves are displayed in Figure 1B.
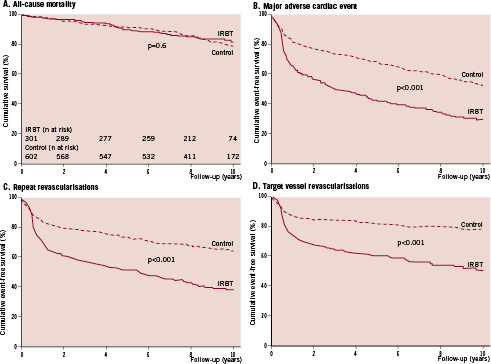
Figure 1. Cumulative probability of event-free survival. The following endpoints were used in the Kaplan-Meier event-free survival curves: all-cause mortality (A); composite patient oriented endpoint of major adverse cardiac events, which included all-cause mortality, any MI and any repeat revascularisation (B); repeat revascularisation (C); and target vessel revascularisation (TVR) (D). IRBT: intracoronary radiation brachytherapy
At five months, the cumulative MACE-free survival was still similar among IRBT patients and control patients (IRBT 89% versus control 90%, log rank p=0.5). Between five months and two years of follow-up, IRBT patients had increased MACE as compared to the control patients (p<0.001) (Figure 2).
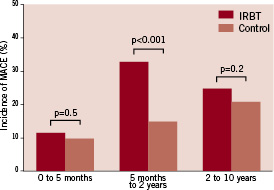
Figure 2. Incidence of MACE during various follow-up periods. IRBT: intracoronary radiation brachytherapy; MACE: major adverse cardiac event
At two years, cumulative MACE-free survival was 56% in the IRBT group and 76% in the control group (log-rank p<0.001). The differences persisted throughout the follow-up period. Cumulative 10-year MACE-free survival rate was 29% in the IRBT group and 52% in the control group (log rank p<0.001).
After adjustment, IRBT was associated with a higher MACE rate (adjusted HR 1.8, 95% CI 1.5-2.2). Moreover, IRBT was associated with higher rates of MI (adjusted HR 3.9, 95% CI 2.3-6.5), repeat revascularisation (adjusted HR 1.9, 95% CI 1.5-2.3), and TVR (adjusted HR 2.2, 95% CI 1.7-2.8). The difference in MACE rate though, was mainly driven by repeat revascularisation (Figure 1C), which especially consisted of TVR (Figure 1D). Furthermore, two or more repeat TVRs were performed in 36 patients (12%) in the IRBT group compared to 35 patients (6%) in the control group (p<0.01).
Subgroup analysis
After stratifying the study population by the indication for PCI, the two acquired subgroups (treatment for de novo lesions and treatment for restenosis) were evaluated separately. Clinical outcomes of these subgroups are presented in Table 3. In both subgroups, the all-cause mortality after IRBT was similar as compared to standard routine PCI. Adjusted hazard ratios for IRBT-associated all-cause mortality after treatment for de novo lesions and after treatment for restenosis were HR 1.3 (95% CI 0.8-2.0) and HR 0.6 (95% CI 0.4-1.2), respectively. For both subgroups, the Kaplan-Meier cumulative MACE-free survival curves are shown in Figure 3.
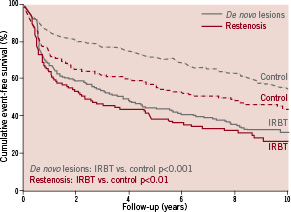
Figure 3. Cumulative probability of MACE-free survival after treatment for de novo lesions or restenosis. Kaplan-Meier MACE-free survival curves were stratified by treatment for de novo lesions (grey) or treatment for restenosis (red). MACE was defined as all-cause mortality, any MI and any repeat revascularisation. IRBT: intracoronary radiation brachytherapy; MACE: major adverse cardiac event
Overall, the groups treated for restenosis seem to have less favourable MACE-free survival curves than the groups treated for de novo lesions. IRBT was associated with higher rate of MACE as compared to standard routine PCI in both treatment for de novo lesions (adjusted HR 2.2, 95% CI 1.7-2.8) as well as in treatment for restenosis (adjusted HR 1.4, 95% CI 1.0-1.9).
Discussion
The objective of this study was to evaluate the late clinical outcome and potential very late adverse effects after intracoronary β radiation brachytherapy in comparison to a propensity score matched control group treated with standard routine PCI. Our main findings were that although IRBT patients had similar mortality rates as the control group, an increased rate of MACE was observed between five months and two years after IRBT. This increase in MACE was mainly driven by repeat revascularisations, which especially consisted of TVR. Between two and 10 years of follow-up the event rates were similar between the two groups.
Efficacy of IRBT
Several randomised trials have initially reported the effectiveness of IRBT for treatment of in-stent restenosis at the short-term. Patients treated with intracoronary β radiation had less binary angiographical restenosis in the β radiation arm of the Washington Radiation for In-Stent Restenosis Trial (BETA-WRIST) at six months (IRBT 34% versus control 71%, p<0.001), the Stents and Radiation Therapy (START) trial at eight months (IRBT 29% versus control 45%, p<0.001), and the Intimal Hyperplasia Inhibition With Beta In-stent Trial (INHIBIT) at nine months (IRBT 26% versus control 52%, p<0.001).5-7 Furthermore, IRBT resulted in less MACE in the BETA-WRIST at six months (IRBT 34% versus control 76%, p<0.001), START at eight months (IRBT 19% versus control 29%, p<0.05), and INHIBIT at nine months (IRBT 15% versus control 31%, p<0.001).5-7 Comparable effectiveness of γ radiation was shown by the Scripps Coronary Radiation to Inhibit Proliferative Post-Stenting (SCRIPPS) trial, the Washington Radiation for In-Stent Restenosis Trial (WRIST), and the Gamma-One Trial, reporting lower rates of clinical and angiographical restenosis at six to nine months after IRBT with γ irradiation.2-4 In our study, similar MACE was observed at five months after IRBT as compared to the control group.
The SCRIPPS, Gamma-One and START reported that the initial beneficial effects of both β and γ radiation on MACE was maintained at two to three years of follow-up.19,20,31 However, in the SCRIPPS, late angiography demonstrated a reduction in lumen diameter between six months and three years in the IRBT patients but not in the placebo patients.20 Furthermore, an increased rate of target lesion revascularisation was observed beyond six months after IRBT.20,23 Our study reports an increase in MACE between five months and two years after IRBT. This is in line with the results of the SCRIPPS and Gamma-One trial, but seems to disagree with the START. However, the START included patients with smaller lesion lengths and only used smaller source lengths, which may be an explanation for the difference as compared to our study. Furthermore, per protocol angiographic follow-up in our study at six months after IRBT may have led to an increased incidence of revascularisations in the IRBT group in that period.
Long-term effects
To our knowledge, this is the first study presenting a 10-year follow-up of a large cohort of patients treated with IRBT. Long-term clinical results up to five years after IRBT for restenosis are only available for the γ radiation studies. These trials have reported sustained benefit on MACE.21,23 MACE-free survival rate at five years was higher in IRBT patients in the SCRIPPS (IRBT 62% versus control 35%, p<0.05) and in the WRIST (IRBT 54% versus control 31%, p<0.01) trials.21,23 Patients in our study had a different distribution at five years of follow-up than the above mentioned γ radiation trials (IRBT 41% versus control 67%, p<0.001). However, we have to consider that our study population had more de novo lesions and was treated with β radiation. MACE free survival rates between two and 10 years after IRBT were similar as compared to the control group, indicating that there are no long lasting and late adverse effects.
Effectiveness of IRBT in de novo lesions is questionable. Limited data are available for longer-term outcome of IRBT for de novo lesions. In the Beta Energy Restenosis Trial (BERT), the positive remodelling observed at six months was lost at two years with a decrease in minimum lumen diameter, indicating a “late catch-up phenomenon” which resulted in a increased target lesion revascularisation rate up to two years of follow-up.32 Although the BetAce trial reported that repeat TVR was just significantly less needed at six months after IRBT (IRBT 13% versus control 35%, p=0.04), similar incidence of MACE was observed at one year (IRBT 43% versus control 42%, p=0.9) and five years of follow-up (IRBT 57% versus control 55%, p=0.9).12 Despite optimisation of pre-, peri-, and post-procedural factors, the Beta-Radiation Investigation with Direct stenting and Galileo in Europe (BRIDGE) trial reported that the TVR and MACE rates at one year in the IRBT group (20% and 26%, respectively) were higher than in the control group (12% and 17%, respectively).11
Effect of the learning curve
In the early years of the brachytherapy era, incidence of geographical miss and late stent-thrombosis was high.10,13,33,34 Geographical miss was defined as a injured segment of the coronary artery that receives a low dose of radiation, resulting in an increased neointima proliferation which could potentially lead to edge restenosis.13,35 Since IRBT had become an established therapy for restenosis, the interested segments were completely and effectively irradiated by using longer sources or a sequential pull-back technique, resulting in a lower incidence of geographical miss.17,18 In addition, a prolonged intake of clopidogrel for one year after IRBT is currently widely accepted to prevent late vessel occlusion and late stent thrombosis.6,36 In our study, a large number of the study patients were part of trials that were performed in the early years of the brachytherapy era. The observed increase in TVR in our study population between five months and two years of follow-up may mainly be caused by delayed restenosis of the irradiated lesion (“late catch-up phenomenon”), but also by edge restenosis after geographical miss and late thrombosis after IRBT.14 Between two and 10 years of follow-up, the event rates of the IRBT group paralleled those of the control group. This indicates that very late catch-up or thrombosis after IRBT did not occur in excess to the non-IRBT treated patients, which is reassuring.
Small-volume high-dose β radiation therapy
The use of a single fraction of high β radiation dose in IRBT can be considered as an extreme form of hypofractionated radiation therapy (less fractions with higher doses per fraction).37 Generally, the potential disadvantage of hypofractionation is that the higher fraction dose might cause increased rate of late normal tissue toxicity, which may result in increased late adverse effects. However, in IRBT, the normal tissue volume exposed to high radiation dose was minimised by administrating β radiation (characterised by less tissue penetration than γ sources) locally in the coronary artery. Results from our study indicate that there are no late adverse effects after IRBT, which may be explained by above mentioned small-volume high-dose hypofractionation. Safety of small-volume high-dose hypofractionation has also been reported in radiation oncology by using stereotactic body radiation.37,38
Current role of IRBT
Since the drug-eluting stent era, many centres have abandoned IRBT treatment.39 Drug-eluting stents not only have been proven to be very effective in preventing restenosis in de novo lesions,40 but also are superior to conventional techniques (including balloon angioplasty and IRBT) in treatment of patients with bare-metal in-stent restenosis.41-43 Therefore, due to the broad application of drug-eluting stents, the role of IRBT is nowadays very limited. There might be a renewed role for IRBT in the treatment for in-stent restenosis of drug-eluting stents, but data for this type of therapy remain limited.44
Study limitations
Some limitations of our study need to be acknowledged. First, the study is not a randomised clinical trial but an observational study of a propensity-matched cohort. Despite using propensity methodology and multivariable analysis to adjust for as much as possible confounders that may be correlated to study outcomes, we cannot exclude the possibility of residual confounding. However, as can be seen in the baseline characteristics, the matched control cohort was similar to the IRBT cohort. Although the type of lesion (de novo or restenosis) was not included in the propensity score matching model, the study outcomes were evaluated separately in these subgroups to ensure that the observed effects were applicable for the whole study population. Second, a large number of our study patients were part of trials that were performed in the early years of the brachytherapy era. These patients can be considered to have less favourable outcomes, because in the early years the learning curve of IRBT was not yet complete. Thus, caution is urged in extrapolating our outcomes to IRBT in current clinical practice in which pre-, peri-, and post-procedural factors are optimised.17,18 However, results from our study are suitable to improve our understanding of the very late interaction of localised β radiation therapy on vascular response in humans.
Conclusions
Mortality after IRBT was similar to standard routine PCI. However, IRBT was associated with increased MACE between five months and two years of follow-up, which was mainly driven by repeat revascularisations of the target vessel. Beyond the first two years of follow-up, the new event rates of IRBT and standard routine PCI were similar, indicating that there are no very late adverse effects related to IRBT.

