Abstract
This review details the utility of intravascular ultrasound (IVUS) for the management of peripheral artery and venous disease. The purpose of this document is to provide an update in the use of IVUS in peripheral arterial and venous pathology and demonstrate the use of IVUS as a practical diagnostic imaging procedure to evaluate and treat peripheral vascular disorders. IVUS, a diagnostic tool that relies on sound waves to produce precise images of the vessel being evaluated, was originally introduced to the medical community for the purposes of peripheral artery imaging, though it was quickly adapted for coronary interventions with positive outcomes. The utility of IVUS includes vessel measurement, pre- and post-procedural planning, treatment optimisation, and detection of thrombus, dissection or calcium severity. While angiography remains the standard imaging approach during peripheral intervention, multiple observational studies and small prospective trials have shown that in comparison, IVUS provides more accurate imaging detail, which may improve procedural outcomes. IVUS can also address limitations of angiography, including the need to administer contrast medium and eliminate the ambiguity associated with other forms of imaging. This review provides contemporary examples of where IVUS is being used during peripheral intervention as well as representative imaging to serve as a resource for the practising clinician.
Introduction
General benefits and applications of IVUS
While initially showing promise in the peripheral arteries1, the development of evidence establishing intravascular ultrasound (IVUS) use in peripheral arterial and venous intervention has lagged behind that of coronary procedures23456. Recently, however, physicians have increasingly recognised the potential impact of IVUS imaging to improve diagnostic acumen, therapeutic decision-making and outcomes in non-coronary vessels789. This has resulted in innovation in IVUS technology and its increased use in peripheral interventions1011. In this document we aim to review the foundation of IVUS imaging, explore how it gained adoption during percutaneous coronary interventions, describe current applications of IVUS in peripheral vascular interventions and present clinical scenarios in which use of IVUS should be considered.
Basic principles of IVUS
The creation of images with IVUS begins with transmission of an electrical current through a piezoelectric transducer, which causes expansion and contraction of the material, thereby generating sound waves. These waves partially reflect off the tissue and return the ultrasound energy to the transducer, generating an electrical impulse that converts into tomographic images. IVUS generates precise images of the anatomic structure (i.e., artery, vein) being evaluated, but is dependent on both spatial and contrast resolution6. The image is constructed from the acoustic properties of the tissue; therefore, some tissues will reflect more sound waves than they transmit. Two different types of IVUS transducers are currently used for image acquisition: a mechanically rotating transducer and an electronically switched multi-element array system612.
Classic IVUS imaging involves 20-40 MHz transducers with pictures displayed in grayscale. However, current iterations include a broader range of frequencies that can produce higher fidelity images and achieve maximum diameter penetration. Additional imaging display options are now available, such as colour (e.g., ChromaFlo; Philips Volcano), to better delineate intraluminal structures. Virtual histology IVUS (VH-IVUS), utilising a low radiofrequency signal, has also been used to better characterise the histologic composition of the vessel wall and associated plaque.
Commercially available products
Currently, there are multiple IVUS catheters commercially available for use in the lower extremity vasculature. Many of these were first developed for coronary use but can be used to image similarly sized vessels, such as the tibial arteries. These devices have since been iterated to provide a broader field of view for use in larger vessels, such as the iliac veins and inferior vena cava. Examples of several commercially available peripheral IVUS catheters are displayed in Table 1.
Vessel measurements
One of the most important benefits of IVUS is the ability to accurately measure lumen dimensions. Using IVUS, one can precisely determine lumen diameter, lumen cross-sectional area (CSA) and the size of the reference vessel (e.g., the approximate lumen dimension in the absence of disease). Each measurement can be used to grade severity of disease, size interventional devices and optimise implants. However, differences exist in how to best estimate vessel size (in part due to variations in blood vessel type [i.e., artery and vein]), vessel location, and image quality. This is in part due to a lack of universally accepted standards.
In conventional angiography, measurement of vessel dimensions comprises multiple factors. First, the measured structure represents a small portion of the overall image, so even a small inaccuracy in calibration will be magnified and lead to large errors in size determination. In contradistinction, IVUS presents a magnified view of the vessel. The segment(s) being interrogated occupies nearly the entire display screen, rather than a fraction as would be the case in angiography. Second, accurate determination of the size of a three-dimensional (3D) structure from a two-dimensional (2D) image assumes the vessel is perfectly round, which is often false, such as in the case of eccentric vessel configuration or the presence of eccentric plaque. Similarly, lesions arising from anterior-posterior compression are common in the central veins (e.g., left common iliac and left renal vein compression) and are easily missed on single-plane angiography. Third, angiography only displays images of the lumen, where the contrast is seen, but cannot assess the components, structure or morphology; thus, for example, in the case of diffuse disease, angiography can produce inaccurate assessment of the true size of the reference vessel.
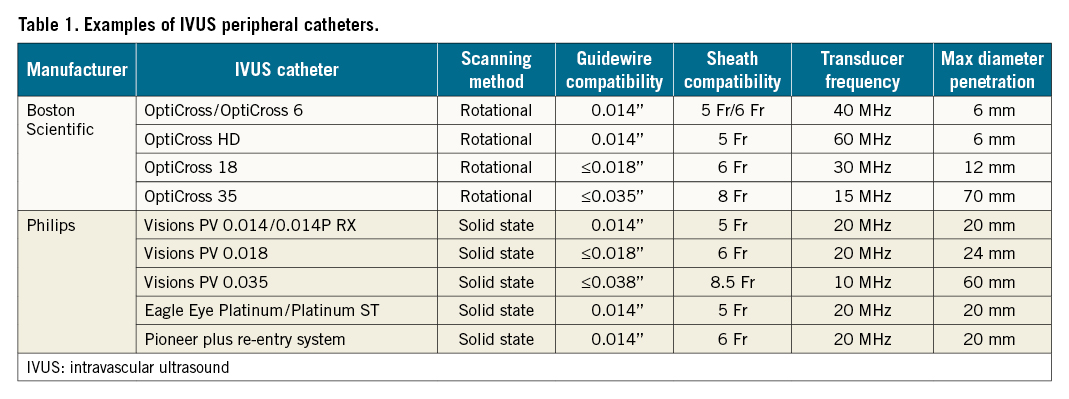
The general approach to measuring a cross-sectional luminal area on IVUS involves identifying the vessel lumen in each segmented axial image, selecting multiple points along the lumen boundary and, then, using the trace function to delineate the vessel lumen (Figure 1). Trace points are automatically or manually selected based on the curvature of the boundary and the local image gradient. The lumen segmentation is complete when the distance between the first and last trace points on the contour is less than the pre-set spacing of the contour points13. Sequential cross-sectional images can then be “stacked” digitally to create a longitudinal view together with the detailed cross-sectional wall anatomy. To obtain the best image quality, the catheter must be parallel to the vessel wall and centred within the lumen; minor angulations can affect image accuracy or cause an elliptical view of the lumen. Similarly, eccentric positioning can distort the representation of the wall and the size of the lumen. Other factors affect image quality and accuracy, including frequency of the wavelength, catheter size, depth of penetration, gain and vessel blood flow. Higher frequency wavelengths will generally enhance image quality/fidelity and delineation of intravascular structures and the vessel wall. However, depth of penetration and the resulting field of view is reduced as frequency increases. Thus, a balance must be struck between the catheter size and the highest available frequency that still allows enough depth of penetration to image the entire cross-sectional area.
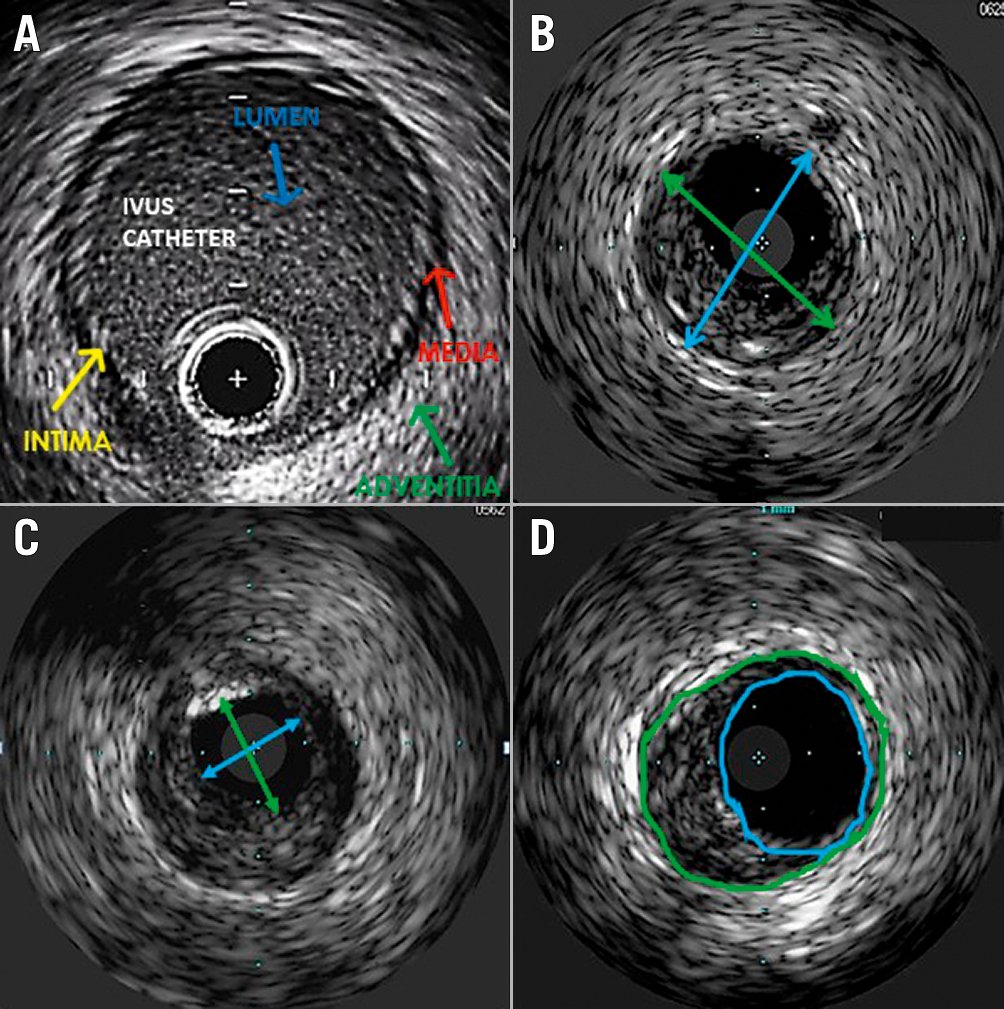
Figure 1. IVUS-derived measurements of the lower extremity artery. A) Layers of arterial wall. Intima: this layer may often not be visible with IVUS due to its thinness; media: this layer appears as a dark space as it is made of homogeneous smooth muscle cells and does not reflect ultrasound; adventitia: this layer has “sheets” of collagen that reflect significant ultrasound waves and appears white; B) Maximum vessel diameter: the maximum vessel diameter is made by measuring adventitia to adventitia to obtain the longest diameter through the centre point of the lumen (arrows); C) Minimum lumen diameter: the minimum lumen diameter is made by measuring the intima to intima to get the shortest diameter through the centre point of the lumen; D) Vessel area and plaque burden: in this panel, tracing of the vessel area inclusive of the plaque (green circle) is compared with tracing of the vessel lumen exclusive of the plaque (blue circle).
Importantly, all vessels include 3 layers: intima, media and adventitia. In muscular arteries, these layers may be more visible, and arteries also have internal and external elastic lamina membranes that can be used for arterial sizing. Unlike arteries, veins contain bicuspid valves formed by folds of endothelium supported by a thin layer of connective tissue. Although 81% of individuals have at least 1 valve in the external iliac-common femoral segment14, they are substantially more common and numerous below the inguinal ligament and must be discerned from other intravascular structures. Differences between the structure of arteries and veins are further delineated in Table 2.
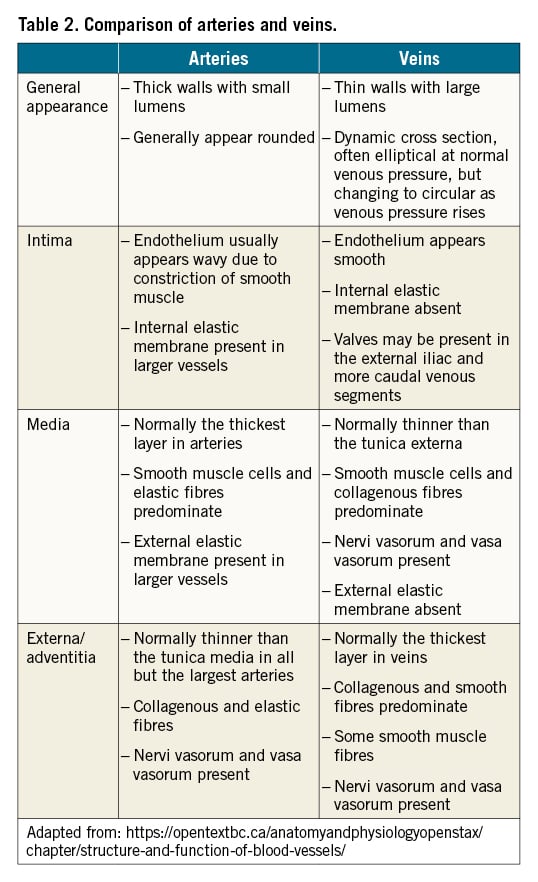
The burden of atheroma can be indirectly measured using IVUS (Figure 1, Central illustration). Plaque burden may be quantified by measuring the plaque plus media CSA and subtracting the luminal CSA. Of note, these measurements may require modification to adjust for vascular remodelling or compensatory enlargement, otherwise known as the Glagov effect15. Calcium can also be readily identified by IVUS (Central illustration). Calcific deposits appear as bright echoes that obstruct ultrasound penetration (also known as “acoustic shadowing”). As IVUS does not penetrate calcium, the thickness of calcium cannot be estimated. However, the circumferential arc of calcium can be measured in degrees. Lastly, metallic stent struts are also highly echogenic, depending upon their thickness and composition. For newly deployed stents, apposition of the stent to the vessel wall can be visualised and quantified (Figure 2). Full apposition is defined as sufficiently close contact to preclude blood flow between any strut and the underlying wall.
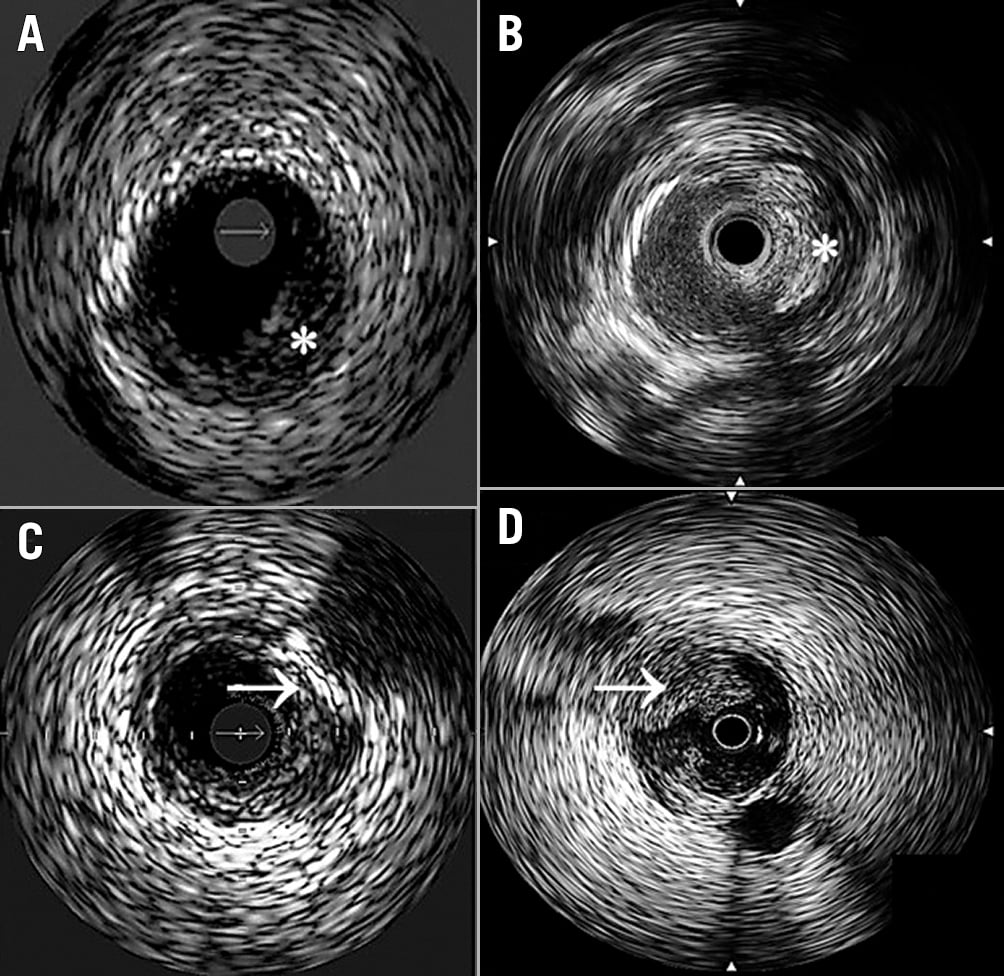
Central illustration. IVUS-guided visualisation of intra-arterial plaque and thrombus. A) Fibro-fatty plaque: echolucent, light grey to grey appearance (*); B) Fibrous plaque: moderately echogenic, light grey to white (*); C) Deep calcific plaque: highly echogenic, white with acoustic shadowing (➔), can grade based on arc of involvement (0-360°); D) Thrombus: echolucent or moderate echogenic, notable encroachment into lumen (➔).
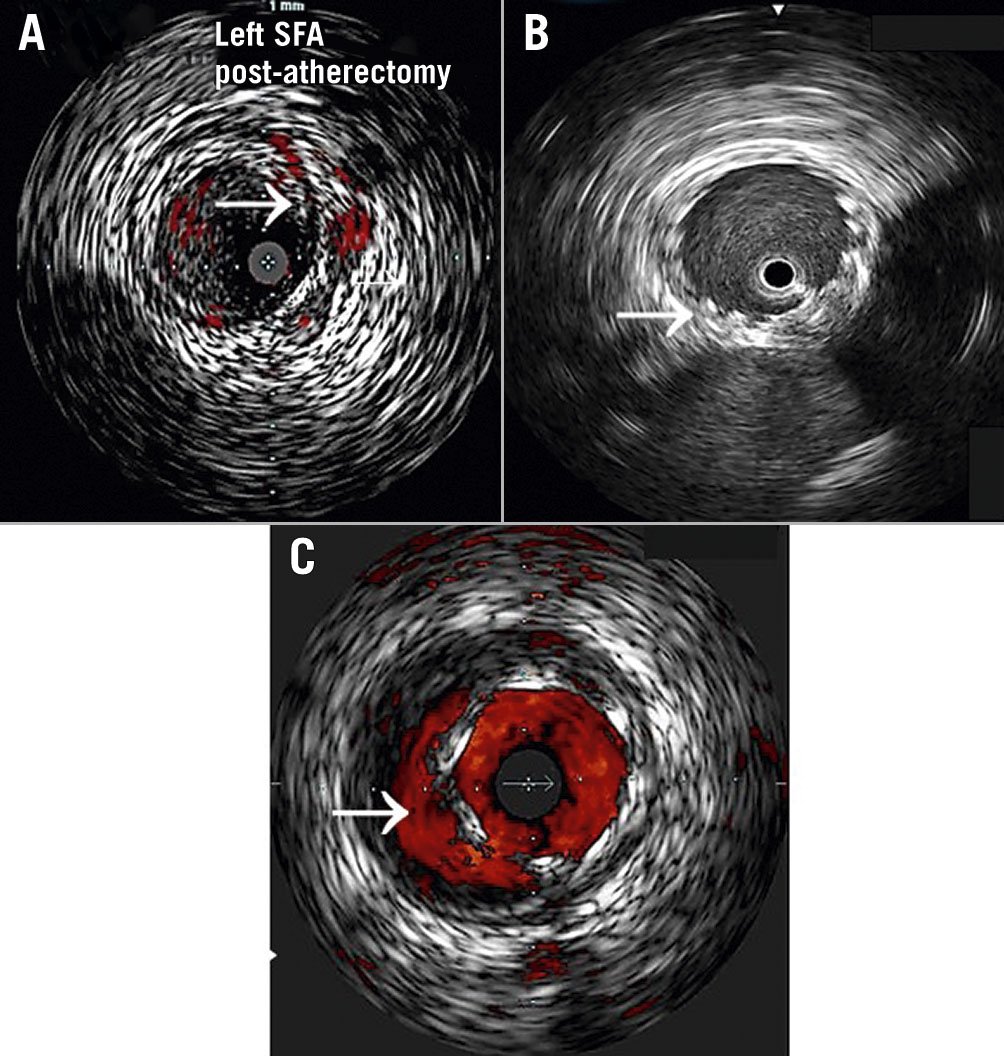
Figure 2. IVUS-guided detection of arterial dissections and stent malapposition. A) Dissection: disruption of vessel intima (➔) following atherectomy. Use of colour (e.g., ChromaFlo) can be helpful to identify false and true lumens; B) Fully apposed stent: the stent appears highly echogenic due to metal struts (➔), which appear bright white with a light fallout behind them. This has a “wagon wheel” appearance. This stent is in contact with the lumen wall circumferentially and appears well expanded (full apposition means stent is in complete contact with the lumen wall. This does not mean that it may not be undersized); C) Stent malapposition: the visualised stent is not in contact with the wall circumferentially, with persistent lumen noted behind stent struts (➔). In this example, ChromaFlo is used to highlight the flow beneath the stent.
Review of the existing evidence base: the coronary IVUS experience
IVUS has been used during percutaneous coronary intervention (PCI) for three decades, primarily as an adjunctive diagnostic device to assess lesion characteristics, severity of disease, lumen dimensions and post-stent apposition. Data from large meta-analyses have demonstrated that assessment by angiography with adjunctive IVUS is superior to angiography alone in reducing adverse outcomes after stent implantation. For instance, in a meta-analysis comparing use of IVUS with angiography in >26,000 patients undergoing drug-eluting stent implantation, IVUS guidance was associated with a significantly decreased risk of target lesion revascularisation (TLR), death, myocardial infarction (MI) and stent thrombosis16. A recent retrospective study of 105,787 Medicare patients undergoing IVUS-guided PCI provided further evidence of benefit, demonstrating that IVUS use was associated with significantly lower mortality, MI and repeat revascularisation, both at 1 year and at median follow-up (3.7 years)17. Coronary IVUS has also been shown to be safe, with few procedural complications and no long-term associated risks181920. This compelling body of evidence has led to the incorporation of IVUS imaging into coronary interventional guidelines, receiving a Class IIa recommendation for assessment of left main coronary artery (LMCA) disease, and a Class IIb recommendation for assessment of non-LMCA disease, guidance of stent implantation and determination of the mechanism of stent thrombosis21. Beyond the guidelines, IVUS is widely accepted as a modality that provides detail not seen on angiography in ostial lesions, bifurcation lesions, stent interface with adjacent vessel (e.g., presence of edge dissections) and segments with overlapping vessels that are hidden angiographically22. Another useful role of IVUS in coronary intervention is to characterise the mechanisms of in-stent restenosis, such as underexpansion or stent compression.
Limitations of angiography and optical coherence tomography (OCT) in peripheral vessels
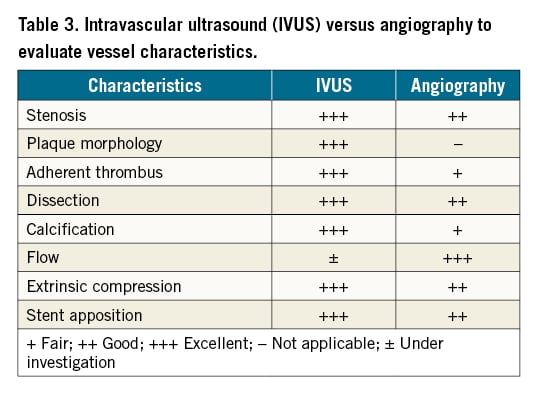
Angiography has traditionally been used for imaging of the peripheral vascular system, providing 2D images of the vessel lumen and its borders. It is most familiar to operators and often required during intervention. However, angiography alone has multiple limitations. First, interpretation of angiographic images may be limited by vessel tortuosity, branch points and concentric lesions23. Second, angiography may underestimate anatomical features, such as lesion length, the presence of diffuse atheroma and disease features (like plaque morphology or presence of dissections and thrombus)724. Third, drawbacks of angiography include increased procedural time, radiation exposure and the need for administration of iodinated contrast25. Table 3 is a summary of the strengths of IVUS compared with angiography to evaluate peripheral vascular disease.
OCT, while an emerging imaging modality in the coronary space, has limited evidence for use in the periphery. When compared with IVUS, OCT may be able to visualise plaque and vessel characteristics with higher resolution and provide longer pull-back lengths at faster speeds26. However, its use is restricted due to the requirement for contrast injection to clear the blood from the vessel lumen. This compromises the ability to acquire OCT images in larger vessels, in particular those >5 mm in diameter27, and creates liability among patients with renal insufficiency. However, certain groups have used non-contrast solutions with some success2728.
Limitations of peripheral IVUS
IVUS also has limitations which may influence its use. IVUS can be complex to set up and interpret for operators who are not familiar with this technology. This can result in longer procedure times and heterogeneity in how image interpretation influences subsequent treatment. Furthermore, catheter delivery can be challenging when using contralateral access or crossing severely stenosed or long lesions. Lastly, reimbursement for IVUS varies by country and may not cover the full costs of this technology.
Analysis of current utilisation of IVUS in non-coronary vessels
Within the lower extremity vasculature, IVUS can play a multifaceted role in peripheral interventions. Below, the current use of IVUS during both peripheral arterial and venous interventions and the evidence to support its use will be discussed in detail.
Peripheral artery disease
Diagnosis
Plaque morphology (Central illustration)
IVUS is useful in the evaluation of plaque morphology, which may dictate therapy and predict long-term outcomes. IVUS-detected plaque morphology has been used to evaluate lesion volume and composition, before and after treatment with atherectomy29303132, and to predict risk of amputation33. With image processing tools that translate echogenicity into profiles for different tissues, IVUS can subclassify plaques into 4 major categories: 1) fibrous, 2) fibro-fatty, 3) necrotic-lipid and 4) calcific34. As in coronary arteries, IVUS can also be used to interrogate and define suspected lesions that are ambiguous on angiography.
Detection of calcium severity (Central illustration)
IVUS is particularly sensitive in the detection of calcium6 and has a unique role in identifying and grading the degree of vascular calcification. These data may then be used to guide treatment strategies, such as upfront plaque modification with atherectomy or intravascular lithotripsy. IVUS has been used to validate 3 angiographic scoring systems (Peripheral Academic Research Consortium [PARC]35, Peripheral Arterial Calcium Scoring System [PACSS] 36, and the DEFINITIVE Ca++trial)37 (Table 4). Notably, in a study of 47 patients undergoing angiographic and IVUS imaging for assessment of peripheral artery calcification, IVUS detected calcium in 44/47 (93.6%) lesions, whereas angiography alone detected calcium in only 26/47 (55.3%) lesions38. The degree of calcification as evaluated by IVUS has also been shown to predict lumen gain after endovascular therapy of the superficial femoral artery (SFA), with severe calcification in a ≥180° arc associated with suboptimal vessel preparation. In a retrospective review of 130 patients with symptomatic de novo SFA lesions who had successful intervention, IVUS-assessed circumferential distribution of calcium (<180° or ≥180°) was independently associated with lumen gain. The rate of restenosis was higher in patients with calcification ≥180°, and the severity of calcification was associated with the risk of stent malapposition39.
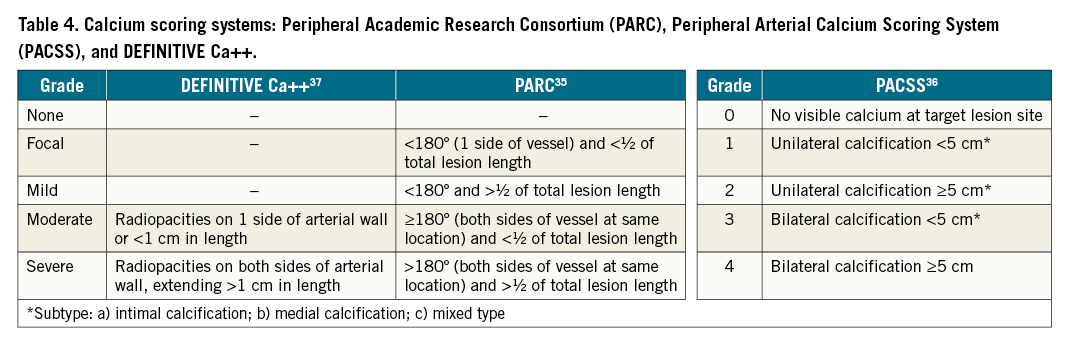
Detection of thrombus (Central illustration)
IVUS is useful in identifying fresh, acute and non-occlusive thrombus because of the high concentration of red blood cells and low fibrin deposition7. In the DETHROMBOSIS study evaluating the role of the invasive treatment of subacute or recently occluded femoropopliteal lesions, 16/17 (94%) patients had thrombus identified by IVUS whereas only 7/17 (41%) patients had thrombus identified on angiography alone40.
Procedural planning (Figure 1)
IVUS can effectively be used for procedural planning to better evaluate lesion characteristics and strategise the optimal approach for treatment, as described above. Furthermore, IVUS has been validated as an effective tool to measure vessel size and length, as well as to evaluate the degree of stenosis both before and after intervention4142. This is notable as angiography alone has been shown to underestimate true lumen size43, which may have a significant impact on long-term outcomes. IVUS can also improve the rate of crossing chronic total occlusions (CTO) by facilitating luminal guidewire crossing and assisting in re-entry techniques444546.
Post-intervention evaluation and optimisation
Dissections (Figure 2)
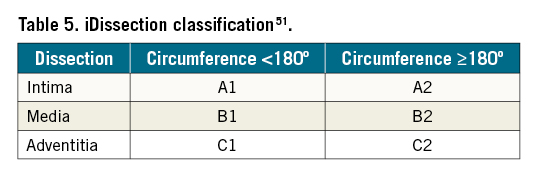
One of the most established roles of IVUS, after peripheral intervention, is identifying and classifying post-treatment arterial dissections47. Traditionally, dissections have been assessed and graded by angiography alone. The iDissection study demonstrated that IVUS identified 4-6 times more dissections in above-the-knee arteries than angiography alone4849. Similar findings have been demonstrated in arteries below the knee50. A proposed IVUS-based classification system categorises the severity of arterial dissections of infrainguinal lesions51 (Table 5). The severity of dissections can then predict short-term outcomes along with long-term patency and may help guide treatment options5253.
Stent optimisation (Figure 2)
IVUS is well established in optimising stent expansion and apposition. In one study, IVUS following procedural completion found that 27% of iliac artery stents were incompletely expanded, poorly apposed or had an associated mechanical disruption despite a normal completion arteriogram54. These findings translate into worse clinical outcomes, both in the short and long term. For instance, in a retrospective study evaluating the primary patency of nitinol femoropopliteal stents placed with versus without IVUS guidance, IVUS-guided stenting was associated with a significantly higher rate of primary patency at 5 years55.
Predictors of long-term outcomes
IVUS may identify factors post-intervention that are associated with greater risks of target lesion failure or stent occlusion. Several studies have demonstrated that following femoropopliteal artery balloon angioplasty, residual stenosis, dissection and lumen area as determined by IVUS are predictive of long-term patency5657. Among patients undergoing stent implantation, IVUS-determined predictors of stent occlusion include minimum stent area, presence of edge dissection and residual plaque burden585960616263.
Peripheral venous disease
In the venous system, IVUS has the greatest utility in the management of obstructive lesions of the iliofemoral venous segment, although it may also be useful in evaluating obstructions of the central upper extremity veins, inferior vena cava and the left renal vein (“nutcracker syndrome”). Chronic obstructive lesions of the inferior vena cava and iliac veins may arise from extrinsic compression by adjacent arterial structures and mass lesions or from post-thrombotic disease. Similar to its use in PAD, IVUS can be used to diagnose, plan and optimise peripheral venous interventions. As the central veins are of substantially larger calibre than the peripheral arteries and large sheaths are required for most interventions, lower-frequency catheters (~10 MHZ) with greater depth penetration are most often utilised.
Diagnosis (Figure 3)
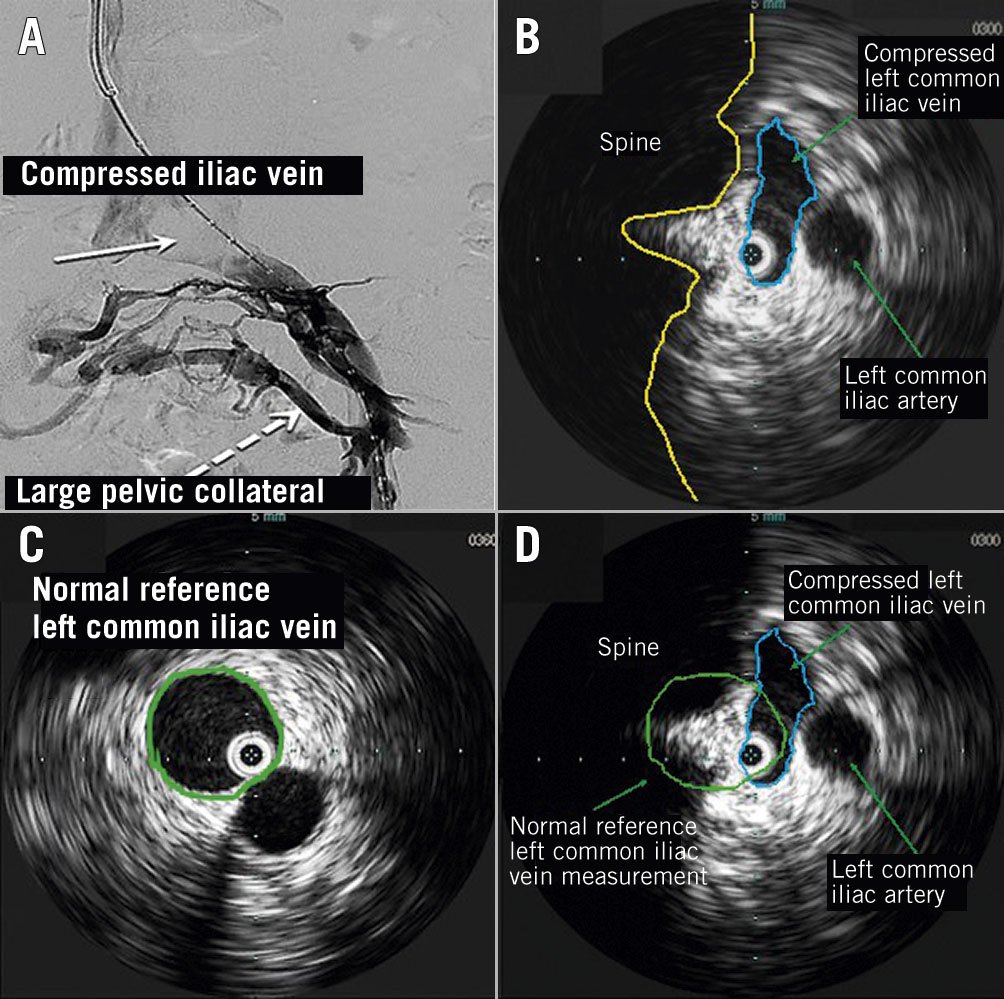
Figure 3. Iliac vein extrinsic compression. IVUS-guidance can be used to evaluate for venous compression and to determine the severity of compression. A) Extrinsic compression of left common iliac vein on venography: lack of contrast flow at site of compression (solid arrow), with prominent pelvic collateral (dotted arrow); B) IVUS determination of aetiology of venous compression: IVUS-guided demonstration of iliac vein compression (blue oval) with parallel view of iliac artery (green arrow) at the site of compression, as seen on venography (Figure 3A, solid arrow). The compressed iliac vein has been traced along the vessel wall to get an estimation of the diameter and area (C) IVUS-guided sizing of the normal reference iliac vein proximal to the stenosis: The reference iliac vein has been traced along the vessel wall to get an estimation of the diameter and area (green circle) proximal to the level of compression; D) IVUS-guided difference between compressed and normal reference veins: the 2 images are superimposed to demonstrate how the differences in areas of the reference and compressed iliac veins can be calculated. The minimum luminal area is divided by the reference luminal area to determine severity of stenosis. As can be seen in the image, there is >50% difference in area estimations suggesting severe compression.
IVUS is considered the “gold standard” for identifying venous iliofemoral lesions64. IVUS is particularly useful in quantifying the degree of venous obstruction, especially from lesions arising from extrinsic compression. Not only do many veins often have an elliptical configuration under conditions of normal pressure, but many compressive lesions also occur in an anterior-posterior plane and are easily missed by single-plane angiography. The severity of such eccentric lesions is better reflected by the cross-sectional area reduction with IVUS than by diameter reduction with angiography64. For example, in a study of 345 consecutive limbs with suspected common iliac vein obstruction, venography underestimated the median degree of stenosis by 30% in comparison with IVUS, and the sensitivity of venography in comparison with IVUS for detection of a greater than 70% stenosis was only 45%65. In another study comparing multiplanar venography and IVUS in 100 patients with advanced chronic venous disease, significant venous obstructive lesions were identified in only 51% of patients by venography versus 81% by IVUS66.
In addition to improved quantification of luminal stenosis, IVUS has further value in identifying intraluminal features not seen on venography, such as mural thickening, residual thrombus, synechia, trabeculation and frozen valves65. A previous deep vein thrombosis (DVT) is suggested by findings of diffuse narrowing, wall thickening or intraluminal echogenic material64.
Although IVUS may be the “gold standard” for identifying venous obstructive lesions, as an isolated measure it is not sufficiently predictive of clinical improvement to warrant intervention based on IVUS alone. Among 68 patients undergoing venous stenting for advanced chronic venous disease, a pre-intervention cross-sectional area reduction of >54% by IVUS best predicted clinical improvement, defined as a >4 point improvement in the revised venous clinical severity score67. However, among the 46 patients undergoing stenting for a cross-sectional area reduction >50% by IVUS, clinical improvement was noted in only 21 patients (positive predictive value 46%). Accordingly, it is difficult to define a threshold stenosis at which a venous stenosis becomes haemodynamically and clinically significant. The determinants of such a “critical” venous stenosis, defined as the degree of stenosis at which upstream pressure increases, are far more complex than in the arterial circulation68.
Procedural planning (Figure 3)
Following diagnosis, IVUS is important in planning venous angioplasty and stenting. Critical components of venous stenting include accurate stent sizing and complete stenting of all diseased areas in which IVUS has significant advantages over conventional venography. With respect to stent sizing, many previous recommendations were based upon the use of Wallstents (Boston Scientific) which often required significant oversizing in comparison to the newly approved dedicated venous stents. Based upon a variety of largely theoretical concerns, optimal venous stent diameters of 16–18 mm, 14 mm, and 12 mm have been recommended in the common iliac, external iliac, and common femoral veins, respectively69. Although such broad recommendations are useful in the setting of chronic venous occlusions, in which case a normal adjacent vessel may be unavailable for measurement, if possible, these recommendations should be interpreted in the context of IVUS-guided measurements of the normal, adjacent reference vessel and stent sizes should be adjusted appropriately. Complete coverage of all disease, without skip areas, is also a fundamental tenet of venous stenting, and IVUS has significant advantages over venography in detecting subtle irregularities of the venous lumen, particularly in the setting of post-thrombotic disease. In a blinded review of 152 patients undergoing endovascular intervention for chronic iliofemoral vein stenosis in 155 limbs, IVUS was used both for disease assessment and determining the optimal landing zones for deep venous stenting. Compared with venography, IVUS was better able to detect maximal area stenosis, anatomic location of maximal stenosis, ilio-caval confluence location and distal landing zones70. This is more accurate than the traditional use of body landmarks with venography to guide venous stenting.
Post-intervention evaluation and optimisation (Figure 4)
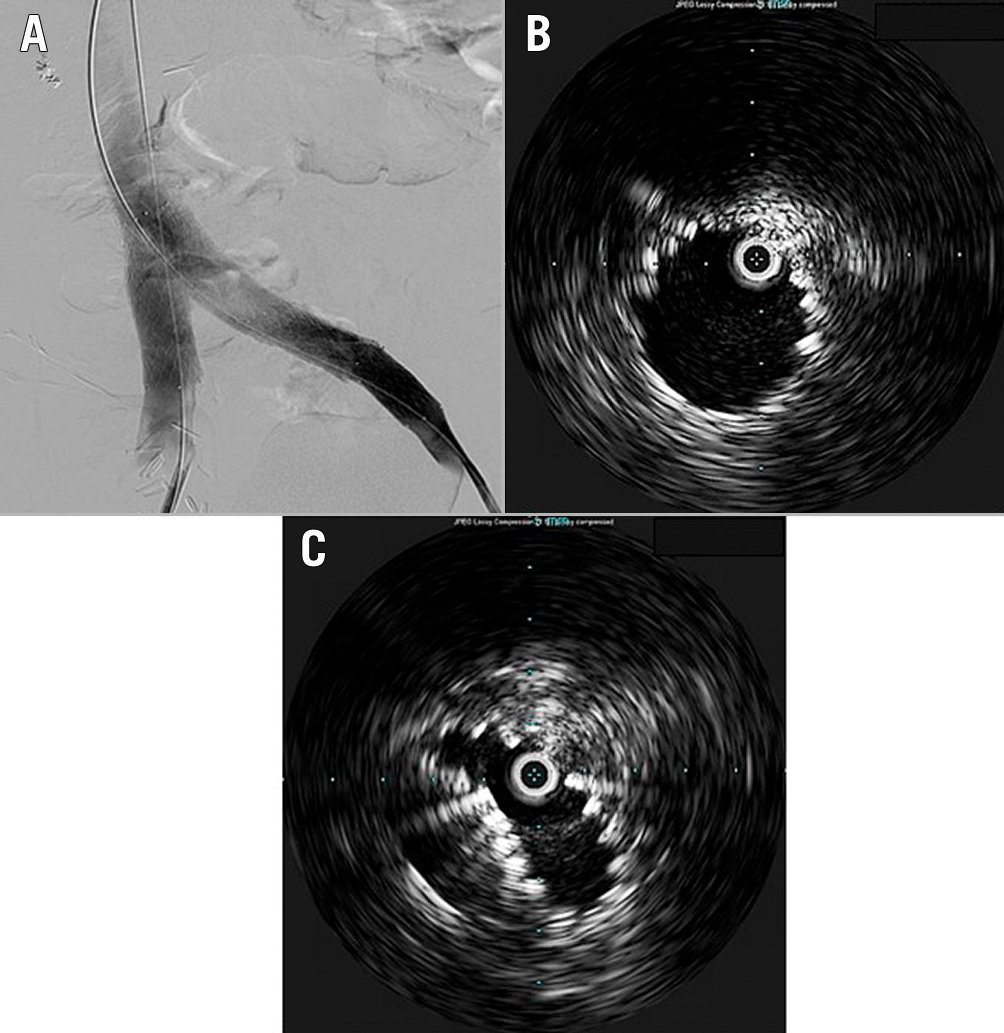
Figure 4. Completion IVUS following bilateral ilio-caval venous stenting can be used to evaluate for optimal stent expansion. A) Venography following bilateral iliac vein stenting; B) IVUS demonstrating well-expanded left iliac vein stent at the left ilio-caval junction; C) IVUS demonstrating right iliac vein stent collapsing due to lack of radial resistive force at the right ilio-caval junction, not readily apparent on venography (Figure 4A).
IVUS may be useful in assessing and optimising the results of venous stenting. Although completion venography is helpful in documenting decompression of venous collaterals, subjective venous flow and resolution of contrast stagnation, IVUS is an invaluable adjunct, allowing assessment of stent sizing, wall apposition and absence of residual compressive or stenotic lesions. IVUS is helpful in visualising areas of residual obstruction that are poorly visualised on venography and may be more accurate than venography in assessing apposition of adjacent stents70. Furthermore, in a study of 274 previously stented limbs undergoing re-stenting of the ilio-caval vein, IVUS-guided overdilation of the stent resulted in significantly better area improvement compared with no overdilation71.
Although IVUS has been most extensively used in the evaluation of chronic venous obstruction, it could have a role in interventional treatment of acute iliofemoral venous thrombosis, in particular in determining device selection. In a study of 33 patients with iliofemoral deep vein thrombosis undergoing pharmacomechanical thrombectomy, IVUS more precisely measured post-procedural segment volume versus venography alone72. Similarly, IVUS distinguished significant residual thrombus, stenosis or May-Thurner anatomy requiring additional interventions in 100% of patients versus 48% evaluated with venography alone72.
The role of IVUS may involve longitudinal evaluation following intervention. Although non-invasive imaging is typically employed first, IVUS can be used when there is ambiguity on imaging in the context of persistent clinical symptoms or if clinical suspicion for device failure is high enough. IVUS has been used in the routine surveillance of stent obstruction in ilio-caval veins73, assessment of midterm patency of stents placed for non-thrombotic iliac vein lesions74 and evaluation of long-term patency of ilio-caval stenting in patients with chronic venous insufficiency75.
Conclusion
IVUS has become an important adjunctive imaging tool during peripheral arterial and venous intervention. Although limited prospective data exist, its safety profile and demonstration of improving procedural outcomes during PCI support its use during peripheral intervention. The current application of peripheral IVUS in clinical practice includes vessel measurement, pre- and post-procedural planning, stent optimisation, and detection of thrombus, dissection or calcium severity. Future work needs to address how to better streamline and implement IVUS use into clinical practice in order to improve rates of adoption.
Acknowledgements
We would like to acknowledge Sahana Natesan, BS, Shylie Ati, BA and Joanna Suomi, AB, MSc for their assistance in preparing and proofing the manuscript.
Funding
E. Secemsky is funded in part by NIH/NHLBI K23HL150290. This review was funded in part by unrestricted educational grants to the Beth Israel Deaconess Medical Center from Boston Scientific and Philips IGT. These companies have not seen or participated in the creation of this manuscript, including the writing of the manuscript, significant edits and the decision whether to submit.
Conflict of interest statement
E. Secemsky is part of the Speakers Bureau, is a consultant for and is on the Advisory Board of Bard, Cook, CSI, Janssen, Medtronic, and Philips; receives grants to his institution from AstraZeneca, Bard, Boston Scientific, Cook, CSI, Medtronic, and Philips. S. Parikh conducts institutional research for and is a DSMB at Boston Scientific; a Global PI at Abbott; and a National PI at Veryan Medical; has received institutional consulting fees from Abiomed, Inari, Penumbra, Terumo, and Canon; and is on the advisory board for Abbott, Boston Scientific, CSI, Medtronic, Philips, and Cordis. He also conducts research for Abbott, Boston Scientific (DSMB), Shockwave, TriReme, and Veryan Medical. M. Kohi is a board member at VIVA Physicians and is a Deputy Editor for the Journal of Vascular and Interventional Radiology. She is also a Global PI of the ELEGANCE registry with Boston Scientific but is not being personally compensated. M. Lichtenberg receives research funding from Cagent Vascular, Biotronik, Boston Scientific, Veryan, plusMedica, Philips, and CR Bard; honoraria for lectures from Cagent Vascular, CR Bard, Boston Scientific, AB Medical, Philips, Terumo, Biotronik, Veryan, and Medtronic; honoraria for advisory board activities from Cagent Vascular, Biotronik, Veryan, Boston Scientific, Philips, Soundbite, Limflow, Covellus, and Medtronic; and participates in clinical trials for Cagent Vascular, Biotronik, Veryan, Boston Scientific, Philips, Limflow, Terumo, and CR Bard. M. Meissner discloses that he has served as a consultant for Cook Medical for a project using a Delphi consensus panel for venous stenting. R. Varcoe consults for Medtronic, Abbott, BD Bard, Philips, Intervene, and Surmodics. A. Holden receives consulting fees as a Medical Advisory Board member from Medtronic, Boston Scientific, Gore, and Philips. M. Jaff is a part-time employee at Boston Scientific; a consultant at Glide Healthcare; and has equity investments in R3 Vascular, Nectero, Vactronix, and Efemoral. D. Chalyan is an employee at Philips IGT. D. Clair has stock options at Nectero Inc.; discloses his involvement with Boston Scientific, Elastimed, Endologix, and Medtronic; and clarifies that he does not receive any income from these relationships as all the income goes to the organisation by whom he is employed. He also serves as a Data and Safety Monitoring Board member for Bard/BD and Boston Scientific where all income goes to the medical organisation. B. Hawkins receives consulting fees from Baim and conducts institutional research for Behring, Hemostemix, and NIH/NHLBI. K. Rosenfield receives grants to his institution from NIH and Boston Scientific; has equity in Accolade, Access Vascular, Althea Medical, Contego, Cruzar Systems, Embolitech, Endospan, InspireMD, JanaCare, Magneto, Neptune Medical, Orchestra, PQ Bypass, Shockwave, Summa Therapeutics, Thrombolex, Truvic, and Valcare; receives consulting fees from and is a board member at Angiodynamics, Boston Scientific, Getinge-Atrium, Contego, Mayo Clinic, Neptune Medical, Philips, Summa Therapeutics, Surmodics, Thrombolex, and Truvic. He is also on the scientific advisory board for Angiodynamics, Boston Scientific, Contego, InspireMD, Janssen, Magneto, Neptune Medical, Philips, Summa Therapeutics, Surmodics, Thrombolex, and Truvic; is a board member of the National PERT ConsortiumTM, a not-for-profit 501c3 organisation dedicated to advancing treatment and improving outcomes in pulmonary embolism; and conducts institutional research for NIH/NHLBI.
Supplementary data
To read the full content of this article, please download the PDF.

