
Abstract
Aims: Patients with cyanotic congenital heart disease (CCHD) have been suggested to develop less atherosclerosis than the general population. This study aimed to evaluate the extent of coronary atherosclerosis in patients with CCHD using intravascular ultrasound (IVUS) and near-infrared spectroscopy (NIRS).
Methods and results: Fifteen patients with CCHD (women, 9; median age, 53 years) and 14 acyanotic controls (women, 6; median age, 53 years) were examined with IVUS-NIRS of the right coronary artery (RCA). The patients with CCHD presented with a larger RCA diameter than the controls (external elastic membrane diameter, 6.1 [4.8-6.7] vs. 4.7 [4.1-5.1] mm, respectively; p=0.01). No difference in area stenosis was found between the patients and the controls (15.8% [12.3-19.7] vs. 15.2% [9.5-18.8]; p=0.87). The presence of lipid by NIRS was noted in 43% of patients with CCHD and in 92% of the controls; however, no differences in total or max 4 mm lipid core burden index (LCBI) or in plasma lipid profile were found.
Conclusions: Patients with CCHD presented with larger coronary arteries than acyanotic controls. No difference in the degree of area stenosis in the coronary arteries was found between the cyanotic and acyanotic patients; however, a lower proportion of patients with CCHD showed a positive LCBI.
Abbreviations
ASD: atrial septal defect
BMI: body mass index
BSA: body surface area
CCHD: cyanotic congenital heart disease
EEM: external elastic membrane
IM: intima-media
IVUS: intravascular ultrasound
LCBI: lipid core burden index
NIRS: near-infrared spectroscopy
PFO: patent foramen ovale
RCA: right coronary artery
Introduction
Atherosclerosis is a major cause of morbidity and mortality in the Western world1. It is a chronic multifactorial inflammatory systemic disease most often affecting large- and medium-sized arteries including the coronary arteries, where it may cause ischaemic heart disease2.
Previous studies have reported that patients with cyanotic congenital heart disease (CCHD) have dilated coronary arteries; however, signs of atherosclerosis on invasive angiographic assessments of the coronary arteries are rare, which is suggested to be related to lower plasma lipid levels than the background population3-8. Conventional coronary angiography, however, only provides a luminogram without depicting the vessel wall itself and is therefore limited in visualising the early stages of atherosclerosis.
Intravascular ultrasound (IVUS) provides cross-sectional views of the coronary arteries, thereby allowing accurate measurements of the different layers of the vessel wall and thus detection of the early stages of atherosclerosis9. Near-infrared spectroscopy (NIRS) is an imaging technique that analyses tissue scattering and light absorption in the near-infrared wavelength region and thus identifies lipid-rich plaques10. Hence, IVUS with NIRS imaging provides a detailed assessment of the atherosclerotic burden of the coronary arteries11.
We speculate that the apparent absence of atherosclerosis on coronary angiography in patients with CCHD could be due to limitations in the true detection of plaque burden in their dilated arteries. This study aimed to assess the prevalence of atherosclerosis in the coronary arteries of patients with CCHD using IVUS and NIRS and to compare these findings with those of an acyanotic control group.
Methods
STUDY POPULATION
Adult patients with CCHD at Rigshospitalet, Copenhagen, or Skejby University Hospital, Aarhus, Denmark, were invited to participate in this study. CCHD was defined as a congenital heart defect resulting in oxygen saturation at rest of <92%. Only clinically stable patients were eligible. The control group consisted of patients referred to Rigshospitalet for closure of a patent foramen ovale (PFO) after cryptogenic stroke or an atrial septal defect (ASD). Study exclusion criteria were age <40 years and previous myocardial infarction/ischaemic heart disease.
This study was conducted in adherence with good clinical practice guidelines and in accordance with the Declaration of Helsinki. The Danish ethics committees approved the study protocol (protocol number: H-1-2014-035). Written informed consent was obtained from all participants before inclusion. All participants were assessed at Rigshospitalet, Denmark.
RISK FACTORS
Data on risk factors for cardiovascular disease, including hypertension, hypercholesterolaemia, diabetes mellitus, and cerebrovascular events, were collected. The participants were classified as “smokers” if they were current or former smokers and as “non-smokers” if they had never smoked.
Clinical examination, including measurement of heart rate, transcutaneous oxygen saturation, body weight, and height, was performed at rest and before the cardiac catheterisation. Body mass index (BMI) and body surface area (BSA) were calculated12,13. Non-fasting blood samples were obtained to determine haematologic and inflammatory markers and to measure lipoproteins.
CORONARY ARTERY ASSESSMENT
After right heart catheterisation under local anaesthesia (either for assessment of pulmonary vascular disease in patients with CCHD or for ASD/PFO device closure in control subjects) was performed, the patients underwent invasive coronary angiography with a 5-6 Fr guiding catheter. Unfractionated heparin was administered according to weight during the procedure (70 IE/kg). Following a coronary angiogram, greyscale IVUS and NIRS were performed in the right coronary artery (RCA) using the TVC Imaging System™ (Infraredx, Inc., Burlington, MA, USA). A 3.2 Fr IVUS-NIRS catheter was advanced over a 0.014-inch guidewire distally in the vessel, and intracoronary nitroglycerine (0.2 mg) was administered. The guide catheter was disengaged, and the IVUS catheter was pulled back to the aorto-ostial junction using an R-100 motorised catheter pullback system (0.5 mm/s). During pullback, greyscale IVUS and NIRS images were recorded. IVUS-NIRS images were analysed using CAAS IntraVascular software (Pie Medical Imaging BV, Maastricht, the Netherlands).
IVUS ANALYSIS
Analysis was initiated 10 mm from the aorto-ostial junction of the RCA and as distal as possible for all participants and was performed at frame level every 0.5 mm by a frame skipping procedure. If the frame was not appropriate for measurement, a proximal or distal 0.1 mm frame was chosen instead. Frame-level analysis for the external elastic membrane (EEM) and lumen included the variables average diameter, cross-sectional area, and eccentricity:
![]()
Intima-media (IM) area and volume were calculated as follows:
![]()
![]()
The degree of area stenosis was defined as the extent of IM occupying the EEM. A stenosis <40% of the lumen area was considered within the normal range14.
![]()
The presence of calcium deposits was evaluated at each frame by visual assessment of bright echoes and acoustic shadowing9.
NIRS ANALYSIS
The likelihood of lipid core plaque was displayed as a chemogram after the pullback. The chemogram algorithm processes the spectroscopic information on a map with a red-to-yellow colour scale to determine the probability of lipid core plaque. Red indicates the lowest probability of lipid core plaque, yellow a probability of a lipid core greater than 0.6 11. Lipid-rich plaque was quantified by the lipid core burden index (LCBI). The LCBI was calculated automatically by the CAAS software as the number of yellow pixels divided by the total valid pixels (red plus yellow) in the region of interest multiplied by 1,000; moreover, LCBI was calculated for each frame. The maximum LCBI in any 4 mm region (maxLCBI-4 mm) of the total region of interest was processed.
STATISTICAL ANALYSIS
Continuous variables were expressed as means±standard deviations (SD) if normally distributed and as medians and interquartile ranges (25th and 75th percentiles) if non-normally distributed. Categorical variables were presented as percentages. For continuous variables, unpaired comparisons between the patients and controls were performed using the Student’s t-test if normally distributed or the Wilcoxon rank-sum test if non-normally distributed. To compare categorical data, the chi-square test or Fisher’s exact test was used. A p-value <5% (two-tailed) was considered statistically significant.
To test for any association between cyanosis parameters (i.e., haematocrit or oxygen saturation) and EEM diameter, univariate regressions were performed. All statistical analyses were conducted using SAS statistical software version 7.1 (SAS Institute, Cary, NC, USA).
Results
Fifteen patients with CCHD and 14 control patients were included. All underwent complete coronary angiography and IVUS-NIRS imaging of the RCA. Demographic data are presented in Table 1. No significant differences in age, gender, BMI/BSA, or smoking status between the two groups were found. A higher proportion of the controls had systemic arterial hypertension (43% vs. 7%; p=0.02). Among the patients with CCHD, the most frequent underlying diagnosis was ventricular septal defect (73%); in the control group, 71% of the patients were referred for PFO closure.
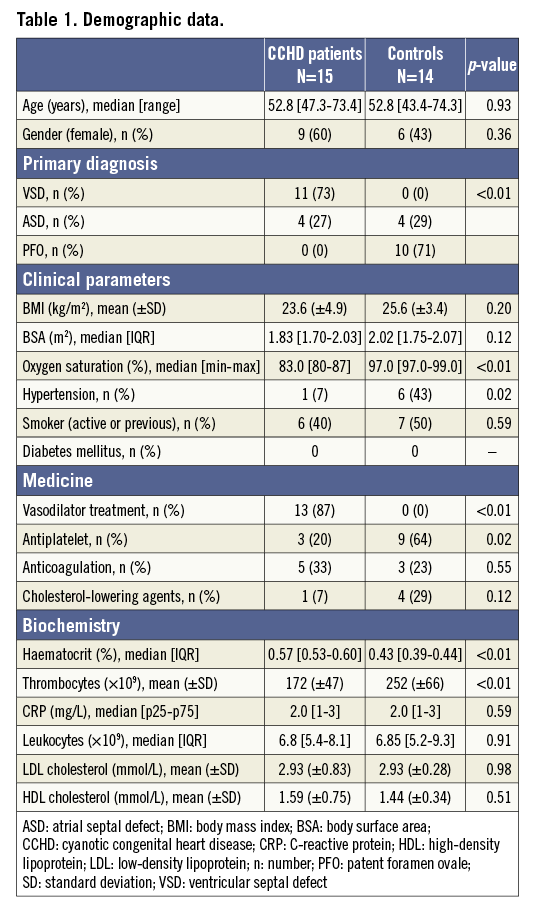
The patients with CCHD had significantly lower oxygen saturation, higher haematocrit, and lower platelet count than the controls. No differences in inflammatory markers (C-reactive protein or leukocytes) or cholesterol levels between the two groups were noted (Table 1). There was no difference in the proportion of CCHD and control patients who received cholesterol-lowering agents (7 vs. 29%; p=0.12).
CORONARY ASSESSMENT
ANGIOGRAPHY
None of the patients in the two groups had evidence of clinically relevant coronary atherosclerosis, which was defined as angiographic stenosis >40% on the angiogram. Patients with CCHD generally exhibited tortuous vessels as well as a high number of collaterals.
IVUS
The longest RCA segment available for IVUS-NIRS analysis in all participants was 46 mm. In one patient with CCHD, 8 mm of the vessel was excluded from the analysis because of poor image quality. Patients with CCHD had a significantly larger lumen area and diameter and a larger EEM area and diameter than the controls (Figure 1, Table 2). The average EEM diameter increased significantly from the most distal part to the most proximal part of the vessel in both groups (patients with CCHD, p<0.001; controls, p=0.003) (Figure 1). No differences in the EEM or lumen eccentricities were observed.
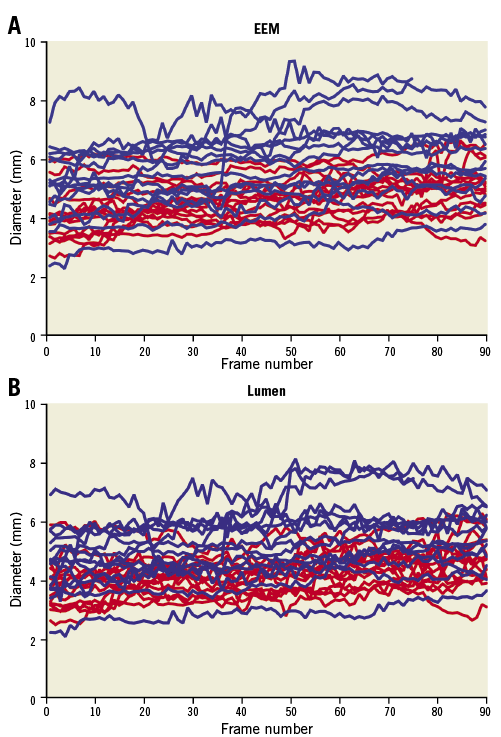
Figure 1. Vessel diameter. Diameter of EEM (A) and lumen (B) measured in millimetres along the vessel. The length of the vessel is given as frame numbers (1-90). Blue line: patients with cyanotic congenital heart disease; red line: controls.
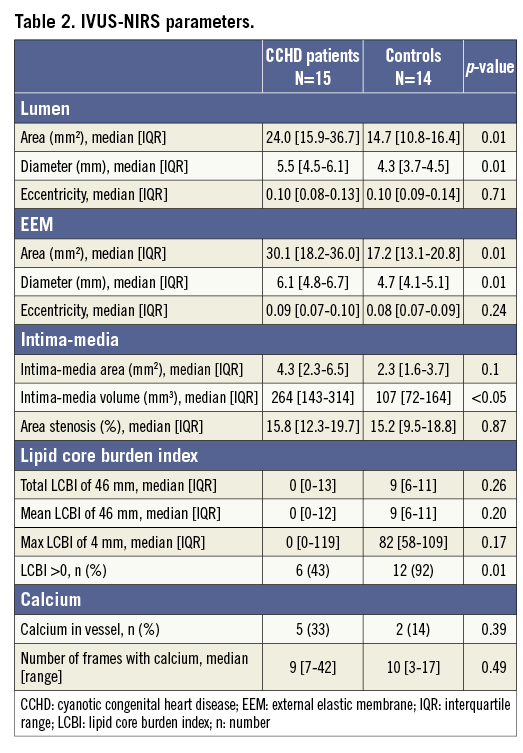
Moreover, a significant association between cyanosis-related parameters (oxygen saturation and haematocrit) and the EEM diameter was found (Figure 2).
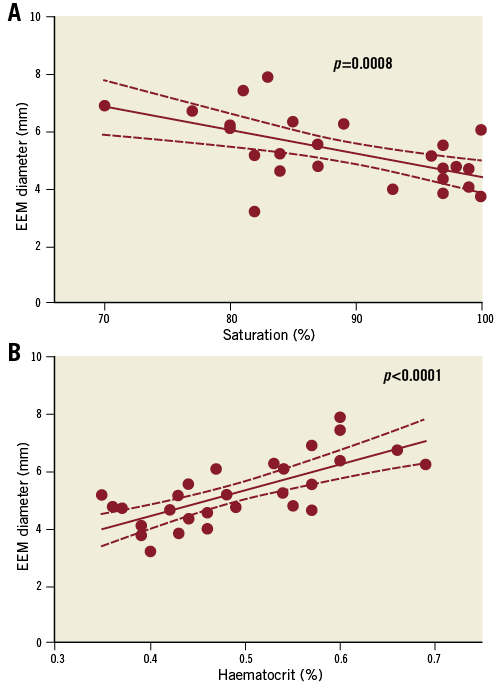
Figure 2. Association between the EEM diameter (mm) and oxygen saturation (A) and haematocrit level (B).
The higher IM volume in the patients with CCHD than that in the controls (264 mm3 [143-314] vs. 107 mm3 [72-164]; p<0.05) was compensated for by the larger diameter of the vessel (Table 2), and no difference in the degree of area stenosis between the two groups was noted (patients with CCHD, 15.8% [12.3-19.7] vs. acyanotic controls, 15.2% [9.5-18.8]; p=0.9).
There was no difference in the prevalence of calcium as assessed with IVUS among CCHD and control patients (33% vs. 14%; p=0.39).
NIRS
No differences were observed in total LCBI and in mean frame-level LCBI between the two groups. Similarly, no difference in maximum (4 mm) LCBI was noted. However, 92% (n=12) of the controls and only 43% (n=6) of the patients with CCHD had a total LCBI >0.
In addition, no association between the total LCBI of the whole 46-mm segment and age, gender, BMI, smoking status, or low-density or high-density lipoprotein was observed.
Discussion
This study examining patients with CCHD using IVUS-NIRS shows that these patients have larger lumens and EEM in the coronary arteries than acyanotic controls. Despite the higher IM volume in patients with CCHD, no differences in the severity of area stenosis were found. Both groups showed a non-clinically significant area stenosis, with an IM volume of 15%. This study also showed that the number of patients with a positive LCBI was lower in the CCHD group than in the acyanotic controls.
Our results confirm the results from previous angiographic studies that the coronary arteries are dilated in patients with CCHD5. However, this is the first study to assess the morphological changes of the coronary arteries by IVUS-NIRS assessment. Increased EEM diameter was present in all the investigated segments without any accompanying focal atherosclerosis, indicating a general rather than a local positive remodelling; the latter is typically seen with atherosclerotic plaque15. Differences in BSA could not explain the difference in EEM diameter. Patients with CCHD have compensatory secondary erythrocytosis due to their chronic low oxygen saturation16-18. The positive association between the EEM diameter and oxygen saturation as well as the haematocrit level suggests that cyanosis and secondary erythrocytosis may play a role in the increased diameter of the coronary arteries in patients with CCHD. Previous studies have also described dilatation of both the retinal and renal vasculatures in patients with CCHD19,20. A common denominator for this widespread vasodilation may well be related to a compensatory increase in nitric oxide synthesis5,19,21.
Despite a significantly higher IM volume in patients with CCHD, both groups presented with a relatively low degree of area stenosis, which was measured by the extent of IM area occupying the “potential” maximum lumen (EEM). Previous studies have argued that stenoses <40-50% are of no clinical relevance and do not affect the lumen by limitation in downstream blood supply14,22.
Furthermore, both groups presented with a relatively low level of calcium deposit, which could be explained by the relatively young age of the patients; calcium deposits are first found in the more advanced stages of atherosclerosis, which are typically observed with increasing age2. The IM proliferation seen in both groups may be characterised as pre-atheroma.
No significant difference in the total LCBI or maximum 4 mm LCBI between the two groups was noted. However, the presence of at least one frame with a probability of lipid by NIRS was observed in 43% of patients with CCHD and in 92% of the controls, although no difference in the lipoprotein profile was found. Atherosclerosis is a chronic inflammatory condition beginning in childhood and takes 20-30 years to develop. It is therefore not surprising that 92% of the acyanotic patients in the control group had lipid deposit in their coronary arteries. Previous autopsy studies showed a high prevalence of atherosclerotic changes in the early ages of healthy men23,24. Moreover, it is therefore highly interesting that fewer than half of the patients with CCHD presented with lipid in the vessel wall. A higher proportion of the controls, however, had lipid-lowering treatment because of their history of cryptogenic stroke, which in turn possibly masked a possible difference in the lipid status. The atherosclerotic process is thought to begin with intimal proliferation, including accumulation of lipid-rich foam cells; thus, because of the low prevalence of vessel lipid at the NIRS assessment, the measured area stenosis (IM tissue) in the patients with CCHD may not represent classic atherogenesis with lipid accumulation2.
Increased vessel diameter in patients with CCHD could result in increased wall tension (Laplace’s law). The observed increase in wall thickness (expressed as IM area) in this study could therefore represent a compensatory response to the increased wall tension25. Further, a small necropsy study in patients with CCHD demonstrated a loss of medial smooth muscle cells, increased medial collagen, and focal intimal fibromuscular hyperplasia in the coronary arteries; however, no evidence of atherosclerosis was found26. The IM area of patients with CCHD may therefore represent non-atherosclerotic adaptive intimal and media hyperplasia and not the classic atherosclerotic process with lipid and calcium deposit. We could also speculate that the young age could be the reason for not observing the classic atherosclerosis characteristics with lipid and calcium accumulation in the intima. However, age could not explain the difference in lipid deposit between the two groups. Nevertheless, whether the IM thickening is due to non-atherosclerotic or atherosclerotic proliferation, blood flow may still be limited if the stenosis reaches 40-50%.
The IVUS-NIRS assessment is limited in determining the true histological composition of the stenosis area, and further studies are warranted to elucidate fully the IM volume in the coronary arteries of patients with CCHD and to clarify the true prevalence of atherosclerosis in the coronary arteries9.
The results of the present study show that IVUS-NIRS assessment in patients with CCHD is feasible. However, as in the background population, it must be recommended that patients with CCHD presenting with symptoms of ischaemic heart disease are managed with a non-invasive test before invasive examination is considered.
The results of the present study suggest that IVUS-NIRS assessment is a valuable addition to invasive coronary angiographic assessment in patients with CCHD presenting with symptoms of ischaemic heart disease due to the additional information on area stenosis and lipid deposits.
As previously shown, patients with CCHD present with tortuous vessels. Vessel tortuosity is associated with various conditions including blood flow alterations. The increased shear stress caused by secondary erythrocytosis in patients with CCHD alters the blood flow in the vessels and explains the tortuosity26,27.
Limitations
The results of this study are limited by the small number of patients and controls. Nevertheless, this should be seen in view of the relatively low prevalence of adult patients with CCHD that is accessible for invasive imaging. Further, the controls were patients referred for closure of either PFO after a cryptogenic stroke or ASD and thus may not represent the background population. Although we did not match the controls by specific atherosclerotic parameters, the two groups can be considered comparable, apart from the higher incidence of hypertension in the controls, as no differences in atherosclerotic risk factors (age, gender, smoking status, BMI, or lipid status) between the patients and the controls were noted. The lower prevalence of hypertension in the patients with CCHD could be explained by the high incidence of treatment with endothelin receptor antagonists and phosphodiesterase-5 inhibitors in this group.
The lipid core detection capability of the TVC Imaging System has thus far only been validated in vessels with a diameter ≤3.5 mm. Larger vessel diameter would mean an excessive blood depth and may trigger low signal outliers masked as black areas on the chemogram, providing false negative results. This may, however, also be affected by the optical opacity of the blood (related to haematocrit, LDL, etc.) and the centring of the catheter. Although this constitutes an important limitation of the NIRS assessment, it only affects the false negatives, and may have contributed to a falsely high negative LCBI in the patients with CCHD.
The IVUS-NIRS examination was only performed in the RCA, though a three-vessel examination might have been more effective for the evaluation of the extent of coronary atherosclerosis. However, we have no reason to believe that any differences among the three vessels could explain the findings. If the patients present with signs of atherosclerosis in at least one vessel, this disproves the hypothesis of an atheroprotective mechanism in these patients. We chose to assess the RCA partly because a complication on the left coronary tree would have been more harmful than on the right coronary tree, in an asymptomatic patient.
Conclusions
Our study showed that patients with CCHD have more dilated arteries than acyanotic controls. The degree of area stenosis measured by IVUS did not differ between the two groups. Both the patients with CCHD and the acyanotic controls presented with a low degree of stenosis which may be described as pre-atheroma. The relatively low prevalence of positive LCBI in the patients with CCHD possibly indicates a non-atherosclerotic IM proliferation.
| Impact on daily practice Improved treatment options have led to an ageing population of patients with CCHD; however, the patients are often stated to be at reduced risk of developing atherosclerosis. The results of the present study show that patients with CCHD had more dilated coronary arteries than controls. Dilated arteries may challenge the detection of stenosis on angiographic assessments. The patients show the same prevalence of area stenosis and calcium deposit measured by IVUS, although a lower prevalence of vessel wall lipid measured by NIRS compared to controls. Classic atherosclerotic processes may not solely explain the IM changes in the coronary arteries of CCHD patients. |
Acknowledgements
We thank all the patients for their participation in this study. Further, we thank Bente Andersen and Bettina Løjmand Mark for their skilful contribution during the IVUS-NIRS image acquisition.
Funding
This work was supported by the Research Foundation of the Heart Centre, Rigshospitalet, and The Danish Heart Association [grant numbers 14-R97-A5000-26026, 15-R98-A5054-26033, 16-R99-A5076-26038].
Conflict of interest statement
T. Engstrøm reports personal fees from Boston Scientific, Abbott, Novo, Bayer, and Medtronic, outside the submitted work. M. Radu reports personal fees from St. Jude Medical, outside the submitted work. The other authors have no conflicts of interest to declare.

