Abstract
Aims: This substudy investigated IABP support in large STEMI complicated by persistent ischaemia within the original CRISP-AMI trial.
Methods and results: Patients were included if the ECG at admission showed summed ST deviation (ΣST-D) ≥15 mm and the ECG post PCI showed poor ST resolution (<50%). Endpoints evaluated were all-cause mortality at six months and the composite endpoint of death, cardiogenic shock or new or worsening heart failure at six months. One hundred and forty-nine patients had ΣST-D ≥15 mm (mean ΣST-D 24±8 mm). Of these patients, 36 (24%) showed poor ST resolution (15 patients in the IABP group; 21 patients in the control group). Mean age was 55±11 years, 89% were male. Mean systolic and diastolic blood pressures were 135±31 mmHg and 83±22 mmHg, respectively. The left anterior descending coronary artery was the infarct-related artery in all cases, primary PCI was successful in 94%. At six months, zero patients in the IABP group died versus five patients in the control group (0% versus 24%; p=0.046). There was a trend towards statistical significance in the composite endpoint (one patient [7%] versus seven patients [33%]; p=0.06).
Conclusions: In this substudy, use of IABP was associated with decreased six-month mortality in large STEMI complicated by persistent ischaemia after PCI.
Introduction
Intra-aortic balloon pump (IABP) counterpulsation has been used as a haemodynamic support system for more than four decades1. The IABP inflates and deflates in synchrony with the cardiac cycle, thereby augmenting diastolic aortic pressure and reducing afterload, resulting in increased coronary blood flow and decreased myocardial workload and oxygen demand2,3. The Counterpulsation Reduces Infarct Size Pre-PCI for AMI (CRISP-AMI) trial hypothesised that IABP as adjunct to revascularisation reduced final infarct size in patients with anterior wall STEMI without shock4. It was a multicentre randomised trial, including 337 patients who were randomised to receive either IABP before primary PCI, or PCI alone. The primary endpoint, final infarct size as assessed by cardiac MRI, showed no difference between the two groups and a trend towards larger infarctions in the IABP group (42.1% versus 37.5%, p=0.06). All-cause mortality at six months occurred less frequently in the IABP group, although it was not statistically significant (2% versus 5%, p=0.12). The exploratory composite endpoint of death, shock, or new or worsening heart failure did reach statistical significance in favour of the IABP group (5% versus 12%, p=0.03).
Interpretation of the ECG plays a fundamental role in the assessment of patients presenting with acute myocardial infarction. Not only the extent of ST elevation, but also the presence of ST resolution provides important prognostic information5,6. Early regression of ST deviation is associated with lower risk of death, recurrent ischaemia, reinfarction and congestive heart failure, while poor ST resolution, impaired myocardial blush grade, or impaired TIMI flow after primary PCI (i.e., markers of persistent ischaemia) are associated with a poor prognosis7-9. Earlier studies showed a possible beneficial effect of IABP support in acute myocardial infarction complicated by persistent ischaemia10,11.
In this sub-analysis of the CRISP-AMI trial, we evaluated the effect of IABP on outcome in patients with electrocardiographic signs of large anterior myocardial infarction complicated by persistent ischaemia despite primary PCI, i.e., patients with the worst prognosis.
Methods
The methods used in the original CRISP-AMI trial have been described extensively12. In short, a total of 337 patients were randomised to be treated by primary PCI with or without adjunctive IABP support. If randomised to IABP, the device was implanted prior to the PCI procedure. In the present substudy, we focused on the patients with electrocardiographic signs of large myocardial infarction on the ECG at admission and with signs of persistent ischaemia, i.e., poor ST resolution at ECG post PCI (as described below).
ELECTROCARDIOGRAPHIC ANALYSIS
After manually identifying the J point to the nearest 0.5 mm, ST deviation was measured 40 ms after the J point to the nearest 0.1 mV. The summed ST deviation (ΣST-D) was calculated by adding the sum of ST elevation in the anterior leads (leads V1 to V6, I and aVL) to the sum of ST depression measured in reciprocal leads (leads II, III and aVF), if applicable. ST resolution (ST-R) was defined as the percent reduction in ΣST-D on the ECG post PCI.
All standard 12-lead ECGs were evaluated by an independent experienced cardiologist, blinded to the actual randomised treatment assignment, procedural results, and outcomes. A second independent and experienced cardiologist, who was also blinded to randomisation as well as results, randomly reassessed 10% of the study ECGs as second reader. Agreement by repeated measurement was 95%.
For this substudy, we included those patients with a ΣST-D ≥15 mm at admission. We excluded patients with left bundle branch block at baseline, paced ventricular rhythm, and patients with ΣST-D <15 mm. Patients were categorised based on the degree of ST-R: good ST-R (defined as ≥50%) and poor ST-R (defined as <50%). The latter group was the target population in this sub-analysis (Figure 1).
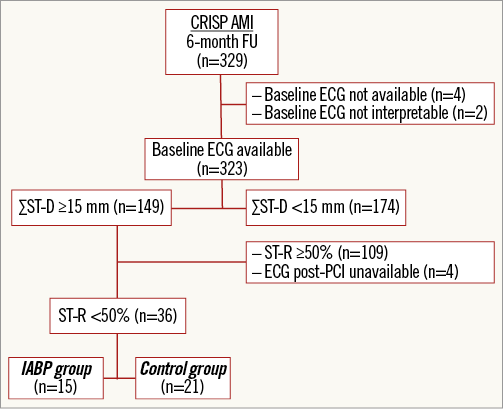
Figure 1. Flow chart of the substudy population. Flow chart of selection of patients in this substudy of the CRISP-AMI trial. ECG: electrocardiogram; FU: follow-up; ΣST-D: summed ST deviation; ST-R: ST resolution
OUTCOME MEASURES
The primary endpoint of the original CRISP-AMI trial was infarct size as a percentage of the total left ventricular mass as measured by cardiac MRI. Primary safety endpoints included all-cause mortality and the rate of major adverse cardiac events including death, repeat myocardial infarction and heart failure at discharge, at 30 days, and at six months.
The present substudy was not pre-specified and not powered for the chosen endpoints.
Our primary clinical endpoints were all-cause mortality at six months, and the composite endpoint of death, cardiogenic shock and new or worsening heart failure at six months. Safety endpoints included major bleeding events according to the Global Use of Strategies To Open Coronary Arteries (GUSTO) trial13. The effects of IABP on infarct size as a percentage of left ventricular mass and left ventricular ejection fraction were not outcome measures in this study because of the possible selection bias due to deaths of patients before undergoing MRI.
STATISTICAL ANALYSIS
Patient characteristics are reported as percentages for discrete variables, while continuous variables are presented as mean±standard deviations. Discrete variables were compared using the χ2 or Fisher’s exact test as appropriate. Continuous variables were compared using the Student’s t-test or Mann-Whitney U test as appropriate.
In correspondence with the original CRISP-AMI trial, Kaplan-Meier analyses and log-rank tests were used to compare time to primary endpoint, and time to composite endpoint of death, cardiogenic shock and new or worsening heart failure. The acquired data were analysed using IBM Statistical Package for Social Sciences (SPSS) for Windows Version 19.0.0.1 (IBM Corporation, Armonk, NY, USA). All statistical tests were two-tailed and a p-value <0.05 was considered statistically significant.
Results
In the CRISP-AMI trial, a total of 329 patients were included in the six-month follow-up (Figure 1). Baseline ECGs were available in 323 patients (98%). In this population, 149 patients were classified as having large anterior wall myocardial infarction with ΣST-D ≥15 mm on the ECG at admission. In these patients, 109 patients were categorised as having good ST-R (more than 50%) and 36 patients as having poor ST-R (less than 50%). In four patients, the ECG post PCI was unavailable. Of those 36 patients with large myocardial infarction and poor ST-R, reflecting persistent ischaemia, 15 patients had been randomised to undergo primary PCI with IABP support, while 21 patients underwent primary PCI alone.
BASELINE AND PROCEDURAL CHARACTERISTICS
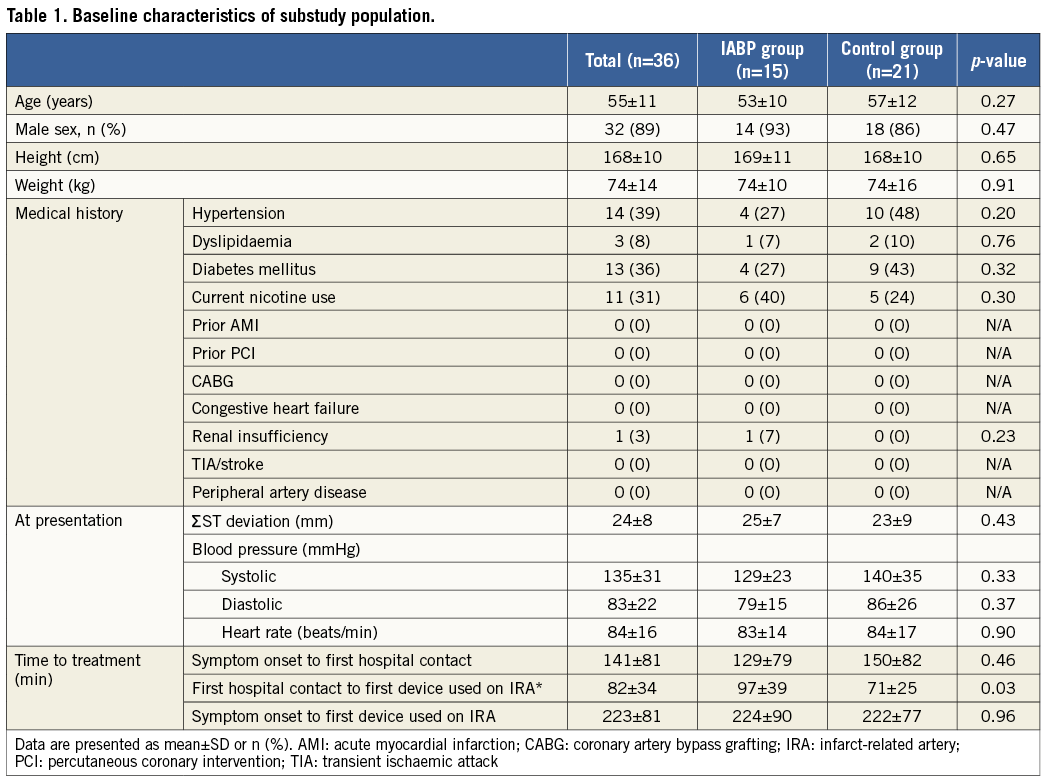
The baseline characteristics of the study cohort of patients with large myocardial infarction and poor ST-R (n=36) are presented in Table 1. Mean age was 55±11 years and 89% were male. At presentation, the mean ΣST-D was 24±8 mm, which was well balanced between the two groups (treated with and without IABP as adjunct to PCI). Systolic blood pressure was 135±31 mmHg on average, while diastolic blood pressure was 83±22 mmHg. Heart rate at presentation was 84±16 beats per minute.
In accordance with the original population of the CRISP-AMI trial, patients were treated in a fast manner with a mean time from first hospital contact to first device used on the infarct-related artery (IRA) of 97±39 minutes in the IABP group versus 71±25 minutes in the control group (p=0.04) (Table 1). This difference was the time used for implanting IABP. There was no difference in the mean time from symptom onset to first hospital contact (129±79 minutes in the IABP group versus 150±82 minutes in the control group, p=0.46). There were no other statistically significant differences in baseline characteristics between the IABP group and the control group, except the previously mentioned time from first hospital contact to first device used on the IRA.
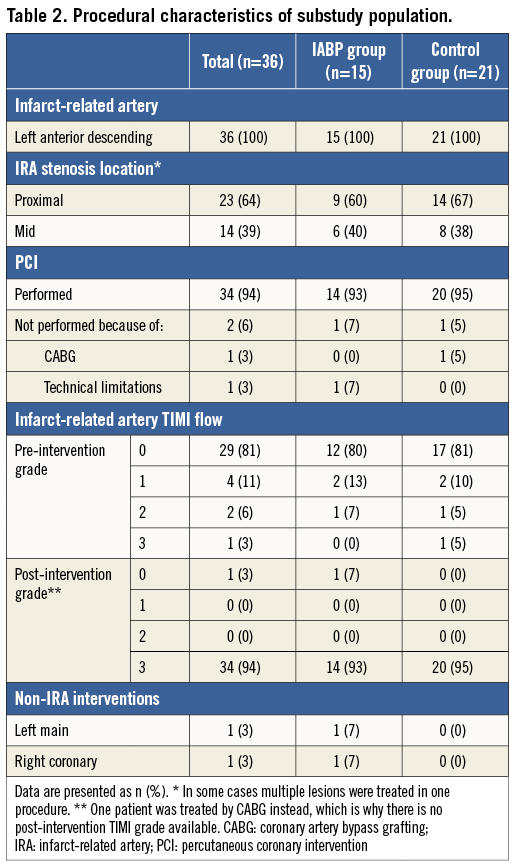
The left anterior descending coronary artery (LAD) was the IRA in all cases, and PCI was performed in 94% of all cases (Table 2). In 81% of the cases, the LAD was totally occluded at the initial angiogram. Primary PCI was successful in 34 patients (94%; post-intervention TIMI flow grade 3). One patient underwent revascularisation with coronary artery bypass grafting instead of PCI.
In the IABP group (n=15), patients were supported by IABP for 22±9 hours. At discharge, patients were treated with aspirin (94%; n=31), clopidogrel (76%; n=25) or prasugrel (24%; n=8), a beta-blocker (82%; n=27), statin therapy (94%; n=31), and either an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker (58%; n=19). There were no differences in medical treatment at discharge between the IABP group and the control group.
There were no bleeding complications in the IABP group. Major bleeding requiring transfusion occurred in one patient in the control group.
OUTCOME
In Figure 2, divided into three panels, are shown the Kaplan-Meier survival curves at six months of the original CRISP-AMI trial population (panel A; N=329; p=0.12), the population with electrocardiographic signs of large anterior wall myocardial infarction (panel B; n=149; p=0.10), and the population of the present substudy (panel C; n=36; p=0.046), respectively. The survival curves diverge most in the subgroup of patients with the worst prognosis. In the population of patients with signs of large anterior wall myocardial infarction complicated by persistent ischaemia, zero patients in the IABP group died versus five patients in the control group at six-month follow-up (0% versus 24%; log-rank p=0.046; panel C). Four patients died before hospital discharge due to refractory cardiogenic shock (n=1) or unsuccessful resuscitation and/or defibrillation (n=3).
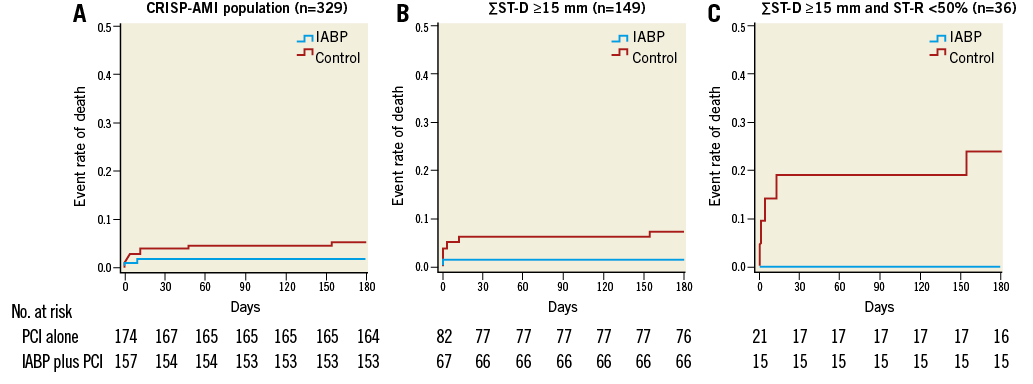
Figure 2. All-cause mortality rate at six months in the different (sub)populations. All-cause mortality rate over six months in all patients receiving IABP plus PCI (blue line) or PCI alone (red line) in the total CRISP-AMI population (three patients vs. nine patients; log-rank p=0.12; panel A); in patients with large myocardial infarction (one patient vs. six patients; log-rank p=0.10; panel B); and in patients with large myocardial infarction and persistent ischaemia (zero patients vs. five patients; log-rank p=0.046; panel C).
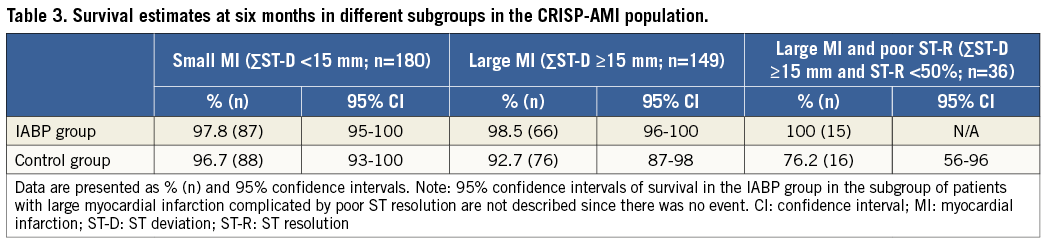
Table 3 shows the survival estimates in different subpopulations within the original CRISP-AMI trial, i.e., small myocardial infarction, large myocardial infarction and large myocardial infarction complicated with persistent ischaemia, respectively. In the IABP group, survival estimates remain constant in the different populations, while in the control group there is a clear decrease in survival in large infarctions, especially when accompanied by persistent ischaemia.
The composite endpoint of death, cardiogenic shock and new or worsening heart failure at six months was reached by one patient in the IABP group and seven patients in the control group and was not statistically significant, although a strong trend was present (7% versus 33%, log-rank p=0.06).
In the control group (21 patients), in five patients (24%) crossover to IABP therapy occurred. Reasons for crossover in these five patients were progression of haemodynamic deterioration in all five patients. There was no crossover from the IABP group to the control group.
An MRI was successfully performed in 28 patients (13 patients in the IABP group [87%], 15 patients in the control group [71%]). Four patients died prior to the MRI procedure (all from the control group) and four patients were unable to tolerate the MRI procedure. The mean infarct size was 43±19% of left ventricular mass (48±24% versus 39±12%, p=0.26). Mean left ventricular ejection fraction on MRI was 41±10%, and not statistically different between the two groups (38±12% versus 44±8%, p=0.13). There was a trend towards less microvascular obstruction in the control group (11.8±8.6% versus 5.4±7.0%, p=0.052). Indirect measures of infarct size, like peak enzymes (CK, CK-MB, and troponin) were available in less than 30% of the study population and therefore not analysed.
Finally, in the population of patients with large myocardial infarction (ΣST-D ≥15 mm; n=149), the presence of poor ST resolution was associated with an increased rate of death (OR 17.419; 95% CI: 2.0-154.7; p=0.01).
Discussion
In this non-pre-specified substudy of the CRISP-AMI trial, intra-aortic balloon pump counterpulsation reduced mortality at six months in patients with large anterior myocardial infarction and persistent ischaemia. The significance was achieved despite the rather small substudy population. There were no complications associated with the use of IABP.
To the best of our knowledge, there are no randomised trials comparing survival in patients with large myocardial infarction and persistent ischaemia treated with IABP. However, the survival rates in this kind of patient treated medically are well described and are comparable to the survival of the control group in this substudy9,14,15. Rezkalla et al previously described a 30-day mortality of 12% in patients with severely impaired myocardial blush grade after PCI for acute myocardial infarction9. In a pre-specified substudy of the APEX-AMI trial, a randomised double-blind placebo-controlled trial on the use of intravenous pexelizumab before primary PCI, the relationship between early ST-segment recovery and outcome was evaluated15. Patients with inadequate ST resolution, analysed with several different methodologies, were at higher risk of death, heart failure or shock up to 90 days after STEMI. The HORIZONS-AMI ECG substudy showed that absent ST resolution 60 minutes post PCI occurred in almost 20% of the patients presenting with STEMI and was associated with a high rate of major adverse cardiovascular events and target vessel revascularisation at three years (29.9% and 20.4%, respectively)14.
In an attempt to find methods to improve outcome in these patients, a retrospective study in a large cohort of patients presenting with acute myocardial infarction reported that IABP support was used to treat persistent ischaemia in 2% of the cases. In these patients, one-year survival was 89%11.
The use of IABP in the presence of persistent ischaemia has not been extensively investigated, but its concept is plausible. The use of IABP is suggested to be effective by, for instance, increasing diastolic aortic pressure, aimed at improving coronary blood flow and myocardial oxygenation. However, with (partially) intact coronary autoregulation, corresponding with the absence of (persistent) ischaemia, it is illusionary to expect that a higher aortic pressure will result in increased coronary blood flow16. On the contrary, in patients presenting with STEMI, adequate epicardial reperfusion, but complicated by persistent ischaemia (or no-reflow) as reflected by insufficient ST-R, coronary autoregulation is completely exhausted.
Persistent ischaemia or no-reflow is caused by a variety of factors, including intramyocardial oedema, spasm, and distal embolisation17. In this situation, myocardial blood flow is proportional to perfusion pressure, which is augmented by the diastolic inflation of the IABP, thereby increasing coronary blood flow and increasing oxygen utilisation by the myocardium. This, in turn, may limit infarct size and improve outcome. This concept, and the increase in myocardial oxygen supply in the presence of exhausted autoregulation, has recently been investigated by simultaneous coronary pressure and flow measurements in humans18.
Over 45% of the CRISP-AMI study population had ST-segment deviation of <15 mm, representing a cohort of relatively small or moderate infarctions with good prognosis anyway7. In fact, in such patients, only a small effect of IABP might be expected, thereby (significantly) decreasing the power of that study.
In the patients with large STEMI, 24% of the patients (n=36) had signs of persistent ischaemia, which is the subgroup in whom benefit can be expected. This is also supported by the numerical survival estimates in Table 3, corroborating the hypothesis that IABP should be reserved for patients with large myocardial infarction and persistent ischaemia, and implies that the outcome of the original CRISP-AMI trial was confounded by the inclusion of a large group of patients with small myocardial infarction.
LIMITATIONS AND UNRESOLVED ISSUES
First of all, this study was a non-powered, non-pre-specified sub-analysis of the original CRISP-AMI trial, implying that these results should be perceived only as hypothesis-generating and viewed with caution. However, as stated, there are few data on how to manage patients with continued evidence of poor reperfusion and ischaemia post primary PCI for STEMI.
Second, a small number of patients did not have both baseline ECGs and ECGs post PCI. These ECGs were missing or ST-R was not interpretable due to conduction abnormalities or arrhythmias. It is unlikely that these excluded patients would have altered the outcome of this study. In only one case, ECG post PCI was not available because the patient died in the catheterisation laboratory (randomised to the control group).
Third, there was a very high crossover rate from the control group to the IABP group in this sub-analysis (24%), due to haemodynamic deterioration and cardiogenic shock. Three of these patients died prior to hospital discharge, while the other two patients reached the six-month follow-up without further events. It is unclear in what way the crossover influenced the results, since the role of IABP support in the treatment of cardiogenic shock is currently under debate. The IABP-SHOCK II trial showed no reduction in short-term and one-year mortality of IABP support in patients with AMI complicated by cardiogenic shock when compared to standard treatment without IABP support19,20. Both in the IABP-SHOCK II trial and in this substudy, asymmetrical crossover to the IABP group was present. One may wonder if an intention-to-treat analysis is suitable in these open, non-blinded studies with a high event rate and considerable asymmetrical crossover. Apparently, the treating physician felt the need to violate protocol and to cross over from control to therapy group, thereby possibly diluting or masking a positive effect of the study device when assessed by intention-to-treat analysis, per-protocol analysis, or as-treated analysis. In the event that this asymmetrical crossover is of influence, it will create a negative bias for IABP, thereby corroborating the results shown in this substudy.
Fourth, comparing MRI results showed no difference in infarct size. However, four patients in the control group died before undergoing MRI. These patients probably suffered from the most severe myocardial infarctions and, by excluding them from the MRI assessment, the average value of infarct size in the control group is underestimated.
Finally, since IABP therapy was randomised and initiated before PCI, IABP support in itself might be responsible for better ST-R at the ECG post PCI. Therefore, the population suffering from persistent ischaemia, had they not received IABP, might in fact have been larger than recorded in this study with 15 patients in the IABP group versus 21 patients in the control group. Also, this might have diluted the beneficial effect of IABP in this study, which is present regardless, and thereby underscores the main finding of this study, i.e., decreased mortality by IABP therapy.
Conclusion
In this substudy in large ST-elevation myocardial infarction complicated by persistent ischaemia after successful primary PCI, the use of IABP decreased mortality at six months. These results mandate further prospective randomised trials as to the beneficial effect of intra-aortic balloon pump counterpulsation in this high-risk population.
| Impact on daily practice In patients admitted with large myocardial infarction, undergoing successful primary percutaneous coronary intervention, but complicated by persistent ischaemia as reflected by insufficient resolution of ST deviation, use of intra-aortic balloon pump counterpulsation significantly reduced mortality in this substudy. Therefore, despite guidelines not to use IABP routinely in acute myocardial infarction, selective use of this support device in these specific patients may be life-saving and should be considered and be readily available in the interventional catheterisation laboratory. |
Funding
This work was supported by the Dutch Technology Foundation STW (Stichting voor de Technische Wetenschappen) under project number 11052. The original CRISP-AMI trial was funded by Maquet (formerly Datascope). The sponsor had no role in the design and conduct of the study, in the collection, analysis and interpretation of the data, or in the preparation, review or approval of the manuscript.
Conflict of interest statement
L. van Nunen has received reimbursement for travel expenses and speaker’s fees from Maquet. M. Patel declares that his institution has received grant funding and reimbursement of travel expenses from Maquet. N. Pijls has received an unrestricted institutional research grant, reimbursements for travel expenses and speaker’s fees from Maquet. The other authors have no conflicts of interest to declare.

